CH2 / methylene insertion to Carbonyl
Mechanism + Description
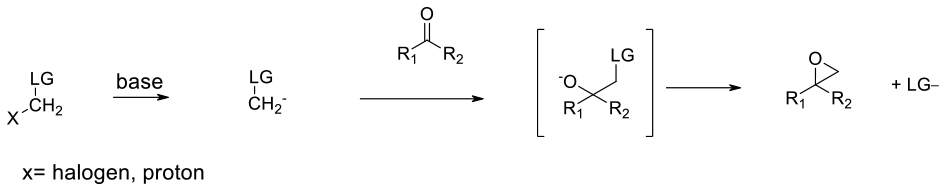
Nuclophilic addition of a methylene anion to a ketone or aldehyde followed by SN2 ring closure
General comments
A methylide (or equivalent) is formed using nBuLi or other strong base, and added into a carbonyl compound. The resultant alkoxide forms the epoxide upon ring closure with the loss of a suitable leaving group. The reaction is applicable to aldehydes and ketones. The reactivity of benzophenone in the reaction suggests that larger groups can be accommodated. Very low temperatures and exothermic reactions may be countered by the use of flow chemistry.
Key references
Chem. Commun. 1988, (5), 333-4. In situ formation and reactions of chloromethyl-lithium under sonochemical conditions
Tetrahedron 2001, 57(43), 8983-8987 An improved preparation of epoxides from carbonyl compounds by using diiodomethane/methyllithium
Relevant scale up example
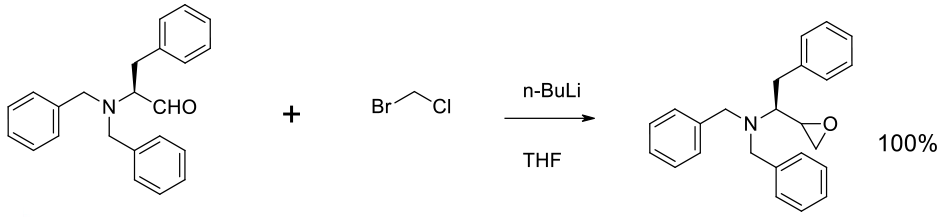
Experimental
190 kg scale
Org. Process Res. Dev. 1997, 1, 45-54
Green Review
-
Atom efficiency (by-products Mwt)
Depends on reagents used. The need for a strong stoichiometric base adds to the poor mass efficiency of this route. - Safety Concerns
Very low temperatures and exothermic reactions may be countered by the use of flow chemistry. Deprotonated halomethanes can decompose to give carbenes. On a large scale, hexyl lithium maybe preferable to butyl Lithium to avoid butane generation. - Toxicity and environmental/aquatic impact
Apart from solvent issues, polyhalogenated alkanes may give issues with release into the aqueous environment or the air. Li and Iodide are of concern in fresh water ecosystems. Alkyl halide synthons are alkylating agents and will give rise to positive PGI alerts. - Cost, availability & sustainable feedstocks
Reagents are generally readily available. - Sustainable implications
Low temperatures and poor mass efficiency. Use of iodide – incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion, although it is possible to recover iodide from waste materials. Li is rated at moderate risk of depletion, Iodine at high risk of depletion.