Corey-Chaykovsky Reaction
Mechanism + Description
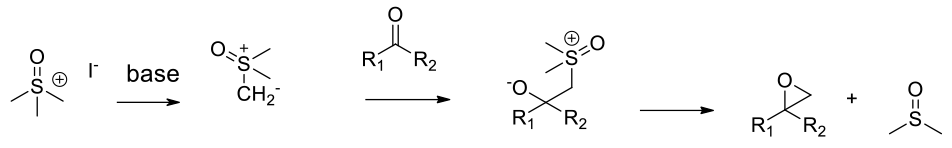
Mechanistically similar to carbonyl Methylene insertion except a stabilised S-ylide is used as the nucleophile
General comments
The Corey-Chaykovsky procedure uses trimethylsulfonium (or sulfoxonium) halide to make the ylide, losing dimethyl sulphide (or DMSO) on ring closure. The reaction is applicable to aldehydes and ketones. There are chiral variants suitable for the synthesis of chiral epoxides.
Key references
J. Am. Chem. Soc., 1965, 87, 1353-1364 Corey-Chaykovsky Reaction
Chem. Commun., 2003, 2644-2651 chiral variants
Advanced Synthesis & Catalysis 2010, 352, 2098-2093 catalytic ylide version of Corey-Chaykovsky
Relevant scale up example
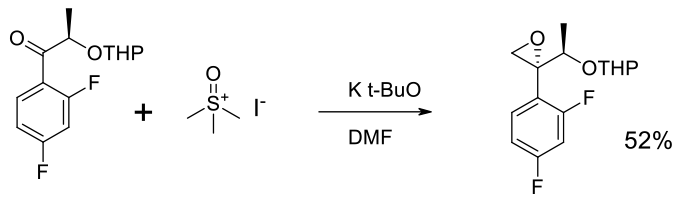
Experimental
20 kg scale
Org. Process Res. Dev. 2013, 17, 658−665
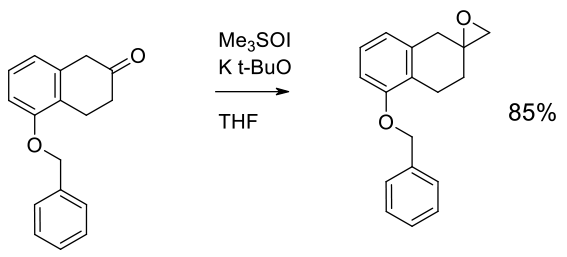
Experimental
70 kg scale
Org. Process Res. Dev. 2009, 13, 716–728
Green Review
-
Atom efficiency (by-products Mwt)
Reasonable atom efficiency generating DMSO or dimethyl sulphide (DMS) by-products plus an inorganic salt. - Safety Concerns
No major hazards identified. The DMS by-product, is an irritant, volatile compound with a disagreeable smell. - Toxicity and environmental/aquatic impact
No major issues – impact of solvents used and any by-product from base used would be major areas for any concern. - Cost, availability & sustainable feedstocks
Reagents available at reasonable cost. - Sustainable implications
Bromide or chloride salts preferred to iodides. Complex Sulfur reagents maybe recovered or the reaction cycle made catalytic.