Dioxiranes and Related Shi Epoxidation
Mechanism + Description
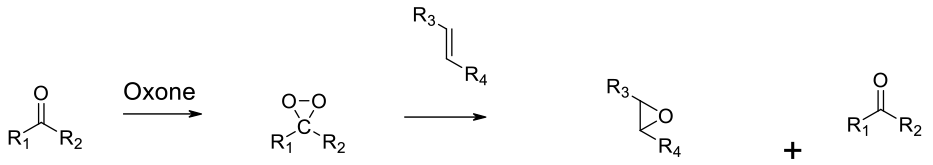
Nuclophilic addition of peroxide to a ketone generates a dioxirane. These react with alkenes at a much faster rate than H2O2.
General comments
Ketones react with persulphate oxidants like Oxone to form dioxiranes. These reagents rapidly transfer oxygen to alkenes to generate epoxides. Simple dioxiranes like dimethyldioxirane can be isolated, but often for safety reasons, these reagents are prepared and used in situ to avoid generating solutions with high concentrations. Dioxiranes derived from chiral ketones can enantioselectively transfer O to alkenes – this is known as the Shi epoxidation. The low temperatures and mild reaction conditions make dioxiranes a good oxidant for the preparation of sensitive epoxides.
Key references
www.chem.wisc.edu/areas/reich/chem547/2-redox%7B22%7D.htm
Org. Process Res. Dev. 2013, 17, 313−316 Practical and Efficient Large-Scale Preparation of Dimethyldioxirane
Org. Process Res. Dev., 2002, 6 405–406 Practical and Environmentally Friendly Epoxidation of Olefins Using Oxone
Chem. Rev. 2008, 108, 3958-3987 Review of Shi chiral epoxidations
J. Org. Chem. 2008, 73, 9539-9543 chiral epoxidation of 1,2’ disubstituted olefins
Synlett, 2010, 2755-27586 . trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane
J. Org. Chem., 2007, 72, 4093-4097 Shi catalyst
Synlett, 2008, 2856-2858 chiral Shi-type catalysts
Relevant scale up example
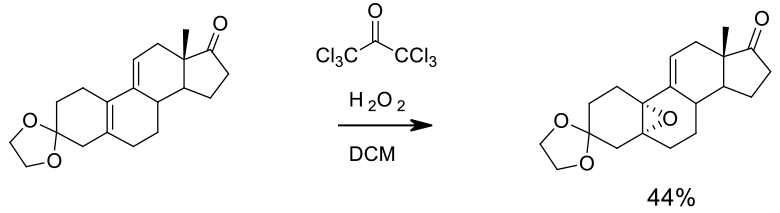
Experimental
46 kg scale
Org. Process Res. Dev. 2002, 6, 20-27
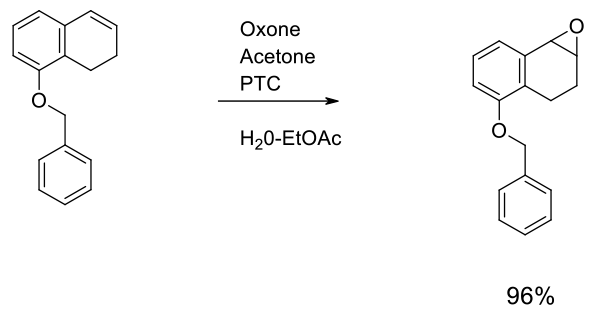
Experimental
Gram scale
Org. Process Res. Dev. 2002, 6, 405-406
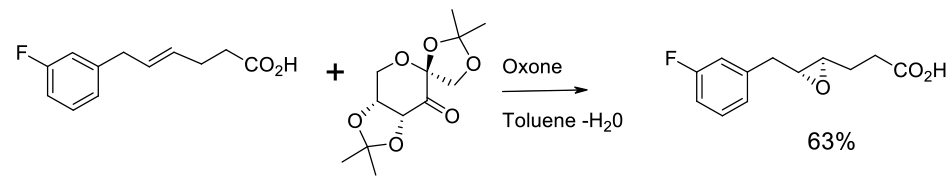
Experimental
30 kg scale
Example of Shi epoxidation
Org. Process Res. Dev. 2007, 11, 44-51
Green Review
-
Atom efficiency (by-products Mwt)
Can be good with low Mwt ketones – acetone. Atom efficiancy decreases with Mwt of ketone. In principal, ketones can be recovered, but this is rarely undertaken. If not recovered, a high Mwt ketone would lead to poor atom economy. With low Mwt ketones like acetone, this process is cheap, and the by-products are innocuous. - Safety Concerns
Dioxiranes are high energy materials and can be explosive when neat/ concentrated. They are best used prepared and used in situ. The safety of oxidation processes run in potentially peroxidisable solvents (e.g. THF, acetone) is dependent on many factors; in these cases, Process Safety should be consulted as soon as possible. Some combinations may generate detonable mixtures or by-products. The work up must ensure that oxidants are removed or destroyed prior to product isolation and waste is treated or appropriately disposed of. To avoid secondary decomposition which may have fatal consequences, it is important to ensure that active oxidants are not present in waste streams. Use of an alternate solvent may avoid such safety concerns. - Toxicity and environmental/aquatic impact
Major concerns here would relate more to solvents used than reagents. Halogenated ketones may give rise to concerns in the aqueous environment. - Cost, availability & sustainable feedstocks
Generally readily available and cheap reagents. Some ketones can be derived from renewables. - Sustainable implications
A reasonable route to epoxides avoiding metal catalysis and mostly environmentally benign reagents. Potential to recover and recycle ketone reagent.