Hydrogen Peroxide with/without Catalyst
Mechanism + Description
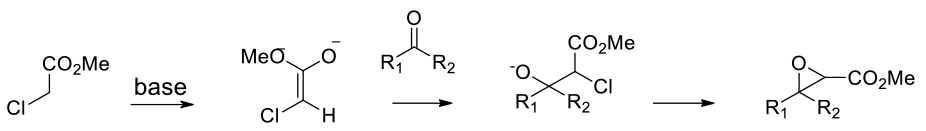
The mechanism varies depending on the alkene – activated alkenes like enones react without a catalyst in a similar fashion to sodium perborate – nuclophilic addition to the alkene followed by loss of H2O. Unactivated alkenes in the presence of metals react via transfer of oxygen from an oxy metal or a cyclic peroxy species.
General comments
Catalytic epoxidation of unactivated alkenes with hydrogen peroxide /metal catalyst is a highly atom efficient primary oxidant that can be used in wide variety of oxidation reactions. Cost of Re reagents may be prohibitive on scale up, but more accessible metals like W and Mn can be used.
The combination of H2O2 and NaOH has been used to epoxidize enones. The combination of H2O2 and nitriles (MeCN, PhCN and CF3CN) have been used to epoxidise isolated C=C bonds, by the in situ formation of the corresponding peroxyimidic acid. The use of various tungsten catalysts have been used in the epoxidation of olefins, however, it should be noted that this combination of reagents could also oxidise alcohol and disulfides. A generally applicable route to epoxides from olefins, but other functional groups may also be oxidised. A number of enantioselective catalysts have been reported that give chiral epoxides with certain classes of alkene e.g. Julia´-Colonna Asymmetric Epoxidation – see references.
Key references
Org. Synth. Coll. Vol. 6, 1976, 679-684; (1976, 55, 52-57). H2O2/NaOH
Org. Synth. Coll. Vol. 7, 1990, 126-130; (1981, 60, 63-67). H2O2/MeCN
J. Mol. Catal. A: Chem., 2004, 218, 13-19 H2O2/RuCl3
J. Mol. Catal., 1994, 86, 243-266. H2O2 MTO Methyltrioxorhenium(VII)
Eur. J. Org. Chem. 2008, 4867-4870 Iron-Catalyzed Epoxidation of Aromatic and Aliphatic Olefins
J. Am. Chem. Soc., 2008, 130, 6070-6071 Chiral primary amine salts catalyze highly enantioselective epoxidations of cyclic enones
J. Am. Chem. Soc., 2002, 124, 11946-11954 Manganese-Catalyzed Epoxidations of Alkenes
Adv. Synth. Catal., 2008, 351, 348-352epoxidation of olefins using hydrogen peroxide as the oxidant in the presence of acetic acid and a manganese catalyst
Org. Process Res. Dev. 2003, 7, 509-513 Development of the Julia´-Colonna Asymmetric Epoxidation Reaction
Supported /Recoverable W catalysts
Org. Process Res. Dev. 2005, 9, 294-296
Org. Process Res. Dev. 2006, 10, 876-880
Org. Process. Res. Dev., 2004, 8, 524-531
Relevant scale up example
Experimental
200 gram scale
Org. Process. Res. Dev. 2010, 14, 72–84
Solid formulations of Hydrogen peroxide
A number of solid formulations of H2O2 exist which can be employed as alternatives to aqueous solutions of H2O2. These formulations can be easier to store and transport, enable accurate dosing of small amounts of peroxide, provide a ‘slow release’ of peroxide when higher concentrations could cause unwanted side reactions. Two commonly used are sodium percarbonate (SPC) and urea:hydrogen peroxide complex. These materials can be used as oxidants on their own, or as terminal oxidants in catalytic processes. Urea hydrogen peroxide is often used as a reagent to Generate peracids from anhydrides in organic solvents.
Mechanism + Description
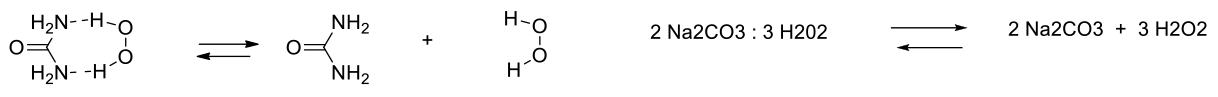
Sodium percarbonate is a perhydrate of Na2CO3
Key references
Chemistry Letters, 1986, pp. 665-666 Sodium Percarbonate (SPC) as a Hydrogen Peroxide Source for Organic Synthesis
J. Org. Chem., 2000, 65, pp 4210-4212 SPC as an Oxygen Source for MTO Catalyzed Epoxidations
Tetrahedron Lett., 2006, 47, pp 99–103 asymmetric epoxidation of α,β-unsaturated aldehydes with chiral amine catalysts
Tetrahedron Letters, 1999, 40, pp 5417-5420 SPC as oxidant for Juliá epoxidation.
Org. Process Res. Dev. 2009, 13, 941-951 urea hydrogen peroxide as the oxidant in Poly-l-leucine-Catalysed Chalcone Epoxidation
Org. Process Res. Dev. 2002, 6, 20-27 epoxidation of a steroid with urea hydrogen peroxide and MTO
Green Review
-
Atom efficiency (by-products Mwt)
Hydrogen peroxide is very atom efficient generating water as a by-product. Urea Hydrogen peroxide and SPC less so, but generate innocuous waste products. - Safety Concerns
Potential for highly exothermic and delayed exotherm reactions so should be scaled with caution. The use of hydrogen peroxide should be limited to 30% wt or less solutions to minimize hazards.
The safety of oxidation processes run in potentially peroxidisable solvents (e.g. THF, acetone) is dependent on many factors; in these cases, Process safety should be consulted as soon as possible. Some combinations may generate detonable mixtures or by-products. The work up must ensure that oxidants are removed or destroyed prior to product isolation and waste is treated or appropriately disposed of. To avoid secondary decomposition which may have fatal consequences, it is important to ensure that active oxidants are not present in waste streams. Use of a alternate solvent may avoid such safety concerns.
Care should be taken with possible catalase –type activity of metal catalysts generating O2 as a side product. - Toxicity and environmental/aquatic impact
Generally good, by-products from H2O2 and excess H2O2 are innocuous to the environment. Any major concerns would come from the solvent used and the potential for any ecotoxic metal catalyst to be discharged into aqueous waste streams. - Cost, availability & sustainable feedstocks
All oxidants readily available and cheap. Precious metal catalyts may add considerable cost e.g. MTO Methyltrioxorhenium(VII) - Sustainable implications
With good catalytic efficiency, and recovery of the catalyst if precious metal, only water is generated as a by-product if H2O2 is used – this reagent is preferred to hydroperoxide oxidants. The use of base metals is preferred to precious metals if at all possible. Re metal has a very high LCI to produce. Metal catalysts like Mn or W are favored. Although very cheap, H2O2 has a reasonable LCI since its manufacture is a complex process ( cannot be produced directly from O2 and H2 ).