Hydroperoxides and Metal Catalysts
Mechanism + Description
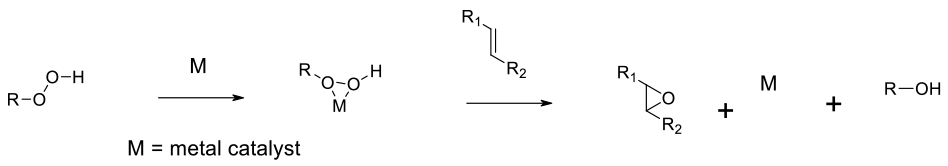
General comments
Alkyl hydroperoxides can epoxidise activated alkenes in the presence of a PTC, and unactivated alkenes in the presence of a metal catalyst. Both homo and heterogeneous catalytic systems have been reported, along with catalysts for chiral epoxidation, many which produce similar results to the Sharpless and Jacobsen AE technologies.
Key references
Catalysis Today 2000, 57(1-2), 87-104 Polymer-supported metal complex alkene epoxidation catalysts
J. Am. Chem. Soc., 2007, 129, 286-287 vanadium-catalyzed asymmetric epoxidation of homoallylic alcohols
J. Am. Chem. Soc., 1958, 80, 5845 t-BuOOH epoxidation of enones
Org. Biomol. Chem., 2004, 2, 1822-1824 chiral epoxidation of α,β-unsaturated ketones
Relevant scale up example
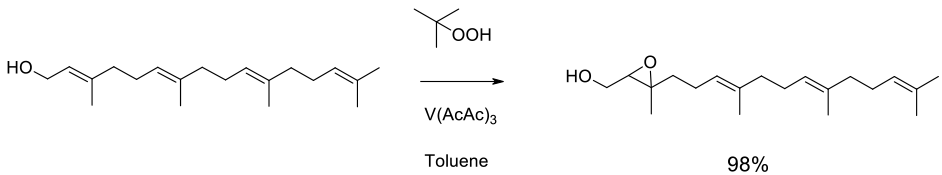
Experimental
Gram scale
Org. Process Res. Dev. 2002, 6, 782-787
Green Review
-
Atom efficiency (by-products Mwt)
Reasonable atom efficiency generating the corresponding alcohol as a by-product. t-Butyl hydroperoxide preferred over cumene hydroperoxide. - Safety Concerns
Potential for highly exothermic and delayed exotherm reactions so should be scaled with caution. Organohydroperoxides are heat and shock sensitive. If using flammable solvents, care should be given to the fact that a flammable and explosive mixture could be generated if O2 is evolved as a side reaction. The safety of oxidation processes run in potentially peroxidisable solvents (e.g. THF, acetone) is dependent on many factors; in these cases, Process Safety should be consulted as soon as possible. Some combinations may generate detonable mixtures or by-products. The work up must ensure that oxidants are removed or destroyed prior to product isolation and waste is treated or appropriately disposed of. To avoid secondary decomposition which may have fatal consequences, it is important to ensure that active oxidants are not present in waste streams. Use of an alternate solvent may avoid such safety concerns. - Toxicity and environmental/aquatic impact
Any major concerns would come from the solvent used and the potential for any ecotoxic metal catalyst to be discharged into aqueous waste streams. - Cost, availability & sustainable feedstocks
Many of the metal catalysts used are abundant, cheap and readily available. - Sustainable implications
The use of base metals is preferred to precious metals if at all possible.