Oxygen and Metal Catalysts/Aldehydes
Mechanism + Description
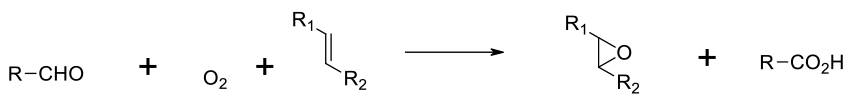
The mechanism of epoxidation in the presence of metals and O2 is probably free radical and while conversions are good, yields can be limited by side reactions. The epoxidation in the presence of aldehydes and O2 is a radical process, with acyl peroxy radials as intermediates.
General comments
Although not widely used in the synthesis of pharmaceuticals, Epoxides can be formed directly from the reaction of O2 and a metal catalyst or aldehydes and O2 – the aldehyde is a stoichiometric reagent.
Key references
Journal of Catalysis Volume 230, Issue 2, 2005, 384–397 Epoxidation of styrene with molecular
Applied Organometallic Chemistry. Volume 26, Issue 5, 252–257, 2012 Epoxidation of styrene with molecular oxygen catalyzed by a novel oxovanadium(IV) catalyst
Chem. Commun., 2013, 49, 1957-1959 Epoxidation of alkenes through oxygen activation over a bifunctional CuO/Al2O3 catalyst
Tetrahedron Lett., 2001, 52, 3489–3491 isobutyraldehyde epoxidation of a variety of alkenes promoted by metal salts such as: Mn(OAc)2·4H2O, FeCl2·4H2O, and Co(OAc)2.
Tetrahedron Lett., 1992, 33, 6827-6830 epoxides from olefins using molecular oxygen in the absence of metal catalysts
Relevant scale up example
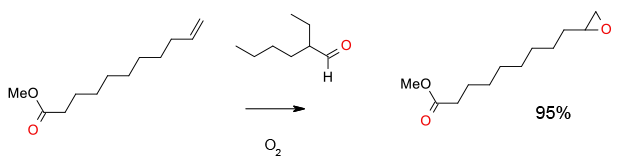
Experimental
2 gram scale
Org. Process Res. Dev. 2007, 11, 44-51
Green Review
-
Atom efficiency (by-products Mwt)
Atom efficiency can be very high with an efficient catalyst and air generating water as a by-product. The use of aldehydes as co-reactants will generate a stoichiometric amount of the corresponding acid as waste, so the Mwt of the aldehyde should be as low as possible. - Safety Concerns
The use of high temperatures and flammable solvents in oxygen containing atmospheres should be given due consideration. The radical nature of the aldehyde reaction may preclude substrates prone to radical catalysed polymerisation. The safety of oxidation processes run in potentially peroxidisable solvents (e.g. THF, acetone) is dependent on many factors; in these cases, Process Safety should be consulted as soon as possible. Some combinations may generate detonable mixtures or by-products. The work up must ensure that oxidants are removed or destroyed prior to product isolation and waste is treated or appropriately disposed of. To avoid secondary decomposition which may have fatal consequences, it is important to ensure that active oxidants are not present in waste streams. Use of an alternate solvent may avoid such safety concerns. - Toxicity and environmental/aquatic impact
Varying metals have different human toxicity and ecotoxicity issues. In all cases the use of catalytic quantities should be beneficial and recovery (and zero release) of precious metals is advised for both environmental and financial reasons. - Cost, availability & sustainable feedstocks
Many of the metal catalysts used are abundant, cheap and readily available. - Sustainable implications
Potentially a very green oxidation technology if the hazards are managed. If possible, base metals should be used as catalysts. Precious metals have a high LCI to produce, and many are on the medium to high risk level for depletion.