Peracids – peracetic, trifluoroacetic acid, mCPBA
Mechanism + Description
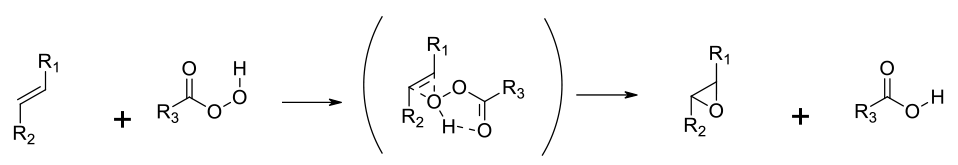
The Prilezhaev Reaction – electrophilic addition of the peroxy oxygen group to the olefin – electron-rich olefins preferred
General comments
A variety of different peracids can be used for the epoxidation of olefins. Probably the most commonly used is mCPBA as it is a readily available, easy to handle solid. It is always supplied as a 70% w/w paste, the other 30% being the parent acid and water, due to its shock sensitivity. Most olefins can be epoxidised with peracid in good to excellent yields. Allylic alcohols, such as cyclohex-2-en-1-ol will undergo stereospecific epoxidation (the hydroxyl group directs the epoxidation). The significant by-product of the reaction is the corresponding carboxylic acid. Whilst chlorinated solvents are commonly used, others such as ethyl acetate have been used as greener alternatives. Magnesium monoperoxyphthalate (MMPA) and trifluroacetic acetic anhydride / Urea H2O2 (TFAA/UHP) have been suggested as an alternatives as they are reportedly less shock sensitive than mCPBA. Epoxidation of alkenes with peracids is generally applicable, however, other functional groups may be oxidised in addition. As well as epoxidation, peracids will oxidise pyridines to the corresponding N-Oxides and sulfide to sulfoxides and sulfones (www.organic-chemistry.org/chemicals/oxidations/meta-chloroperbenzoicacid.shtm)
Generally the reactions scale up well. Base can be added to buffer the acid by-products. However, the potential chemical instabilities and high reactivity of peracids must be considered. In situ generation and consumption of the peracid, with e.g. H2O2, can reduce the concentration of peracid present in the reaction mixture at any time.
Key references
Org. Synth. Coll. Vol. 6, 1988, 862-867 epoxidation with peracetic acid
J. Chem. Soc., 1957, 1958 epoxidation with Perbenzoic acids
Org. Proc. Res. Dev., 2007, 11, 546-559 epoxidation with MMPA
Org. Synth. Coll. Vol. 10, 2004, 704-708 (1998, 75, 153-157) epoxidation with mCPBA
Tetrahedron, 2012, 66, 2455-2462 MMPA epoxidation in the prep of trihydroxysteroids
Org. Proc. Res. Dev., 2003, 7, 839-845 in situ generation of trifluoroperacetic acid
Org. Lett., 2008, 10, 2095-2098 chiral catalyst for peracetic acid epoxidation
Relevant scale up example
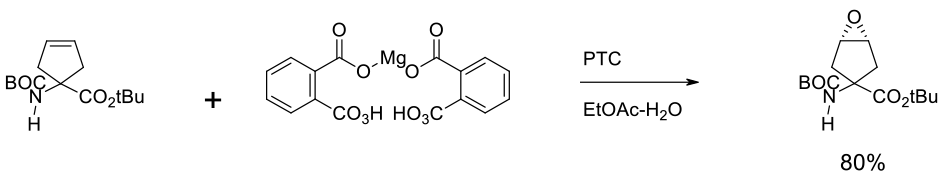
Experimental
11 kg scale
Org. Process Res. Dev. 2007, 11, 546-559
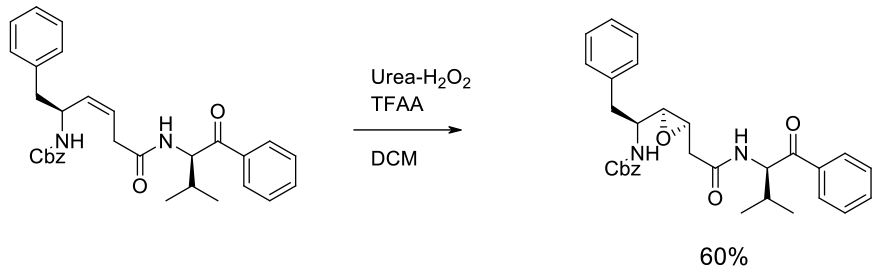
Experimental – in situ generation of CF3CO3H
100 gram scale
Org. Process Res. Dev. 2012, 16, 2031
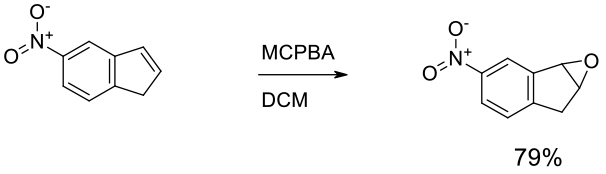
Experimental
7 Kg scale
Org. Process Res. Dev. 2009, 13, 198–208
Green Review
-
Atom efficiency (by-products Mwt)
Can be good with catalytic acid and hydrogen peroxide, by-product water. If using stoichiometric peracids, one eq of acid is produced as a by-product so atom efficiency falls with increasing Mwt. of the acid. - Safety Concerns
With all peracids, there is a risk of explosion and violent reaction with organic materials that support combustion. The safety of oxidation processes run in potentially peroxidisable solvents (e.g. THF, acetone) is dependent on many factors; in these cases, Process Safety should be consulted as soon as possible. Some combinations may generate detonable mixtures or by-products. The work up must ensure that oxidants are removed or destroyed prior to product isolation and waste is treated or appropriately disposed of. To avoid secondary decomposition which may have destructive consequences, it is important to ensure that active oxidants are not present in waste streams. Use of an alternate solvent may avoid such safety concerns. - Toxicity and environmental/aquatic impact
mCPBA is the most commonly used peracid in the epoxidation of olefins. However, the m-chlorobenzoic acid by-product causes some concern in the aqueous environment. Trifluoroacetic acid is also of concern as it is not biodegradable and thus accumulates in the environment. - Cost, availability & sustainable feedstocks
Most peracids are best generated and used in situ – cost and availability mirrors the corresponding acid. Lower Mwt. aliphatic acids can be manufactured from biorenewables - Sustainable implications
Can be reasonable if aliphatic acids are used catalytically with H2O2 (or equivalent) as the terminal oxidant.