Sharpless Asymmetric Epoxidation (AE)
Mechanism + Description
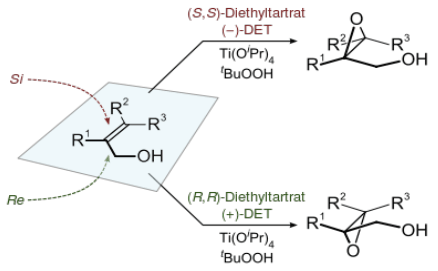
A chiral complex formed between tartrate and Ti containing a hydroperoxide ligand coordinated to the allylic alcohol and delivers ‘O’ to one prochiral face of the alkene.
General comments
Epoxidation of allylic alcohols can be achieved under the original AE conditions of diethyl tartrate (1 eq), titanium isopropoxide (1eq) and tert-butyl hydroperoxide (2 eq) in dichloromethane at –20°C. A further extension to the protocol was the inclusion of molecular sieves, making the reaction catalytic in the amount of tartrate and isopropoxide. The scope of the reaction is limited to allylic alcohols, although variation of R1, R2 and R3 is tolerated.
Key references
J. Am. Chem. Soc., 1980, 102, 5974-5976 Original paper
Science, 1983, 220, 949-951 Synthesis of L-Hexoses
J. Am. Chem. Soc., 1987, 109, 5765-5780 lower catalyst loading
Org. Synth. Coll. Vol. 7, 1990, 461-468 (1985, 63, 66-73) EnantioselectiveEpoxidation of Allylic Alcohols
Relevant scale up example
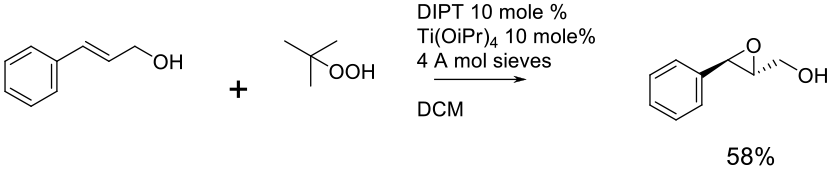
Experimental
150 gram scale
Org. Process Res. Dev. 2007, 11, 354-358
Green Review
-
Atom efficiency (by-products Mwt)
High levels of catalyst complex lead to poor atom economy for the transfer of one atom of O The reaction is often run in the presence of 4A/3A molecular sieves to remove water, which improves the catalytic efficiency of the reaction. - Safety Concerns
Major issues are around the terminal oxidant – t-BuOOH is explosive and shock sensitive. - Toxicity and environmental/aquatic impact
The Sharpless AE is typically carried out in DCM which gives the biggest drawback from an environmental standpoint. - Cost, availability & sustainable feedstocks
Despite moderate catalytic efficiency, all reagents are cheap and readily available - Sustainable implications
Titanium is abundant and rated at low risk of depletion