Acids
Mechanism + Description
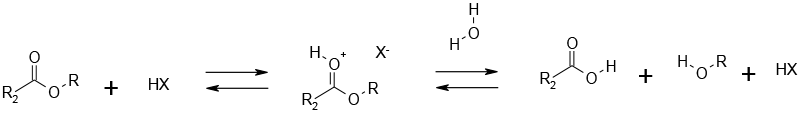
Protonation increases the electrophilicity of the carbonyl group towards water as a nucleophile. Acid cleavage of t-butyl esters follows a pathway via the t-butyl cation.
General comments
Acid catalysis offers another way to accelerate the reaction rate if spontaneous reaction with water is too slow. The hydrolysis of esters for preparative purposes using acidic conditions is usually reserved for base sensitive substrates. As hydrolysis under acidic conditions is an equilibrium process, the reaction will not go to completion unless the alcohol is removed as it is formed or a large molar excess of water is used. Typically a simple inorganic acid is employed as the catalyst – HCl, H2SO4, H3PO4. Where amide hydrolysis is run concurrently with ester hydrolysis, strong HCl (6N) is often the reagent chosen.
Carboxylic acids are often protected by conversion into the corresponding tert-butyl esters, which are highly stable under neutral and basic conditions but which can be hydrolyzed under acidic conditions. A wide range of acids may be used including AcOH, formic, p‑toluenesulfonic, HCl, HBr, sulfuric, trifluoroacetic, triflic, etc. p-toluenesulfonic acid selectively removes t-butyl esters in the presence of benzyloxycarbonyl or trifluoroacetyl groups. AcOH/HBr does not affect phthaloyl groups or trifluoroacetyl groups at 10°C but it will simultaneously remove benzyloxycarbonyl or t-butyloxycarbonyl groups along with t‑butyl esters. Formic acid is suitable for use on b-lactam substrates. Anhydrous HCl/solvent is suitable for use on b-lactam and oxazolidinones. SEE ALSO BOC DEPROTECTION GUIDE.
Key references
Org. Proc. Res. Dev., 2001, 5, 498 use of H2SO4
J. Am. Chem. Soc., 1960, 82, 3359 t-butyl esters of amino acids
Synthetic Commun., 1982, 12, 855 Efficient demethylation of methyl esters with anhydrous trifluoroacetic acid
Org. Proc. Res. Dev., 2008, 12, 168 chemo selective hydrolysis with triflic acid
J. Org. Chem., 1982, 47, 154 Mild and selective cleavage of t-butyl esters
Heterogeneous catalysis
For both acid and base-catalysed hydrolysis, a number of solid phase catalysts that operate heterogeneously have been reported. These can be filtered off after reaction and often reused.
Chem. Rev., 2004, 104, 199
Chem. Rev., 2010, 110, 1
Relevant scale up example
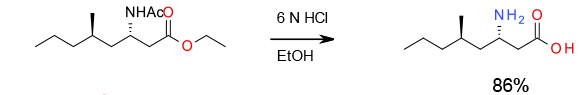
Experimental
100 Kg scale
Org. Process Res. Dev.,2011, 15, 1172
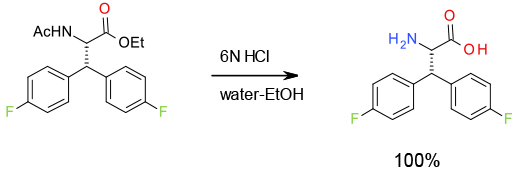
Experimental
300 Kg scale
Org. Process Res. Dev.,2013, 17, 69
Green Review
-
Atom efficiency (by-products Mwt)
Dependent on acid used – most are employed in large excess or as a solvent, if neutralization is required then more acid usually requires more base (unless an organic acid that can be largely removed with an extraction prior to neutralisation). - Safety Concerns
Main issues are around corrosion and handling of strong acids. HCl and HBr may give rise to off gassing of volatile alkyl chlorides/bromides. Acid deprotection of t-butyl esters may give rise to iso-butylene. - Toxicity and environmental/aquatic impact
Most mineral acids pose little problem after neutralization and dilution. High phosphate levels may be regulated due to concerns over eutrophication. Organic sulfonated acids and especially fluorinated materials can be persistent and accumulative in the environment. Heating acids like HCl and HBr in alcohol solvents can generate alkyl halides which are positive PGI alerts. - Cost, availability & sustainable feedstocks
Very cheap and readily available. Some organosulfonates are becoming available prepared from biorenewable sources. - Sustainable implications
Generally good with mineral acids – less so for sulfonate esters and fluorinated acids/sulfonates which will have a bigger LCI in their manufacture and more issues with persistence in the environment.