Bases
Mechanism + Description
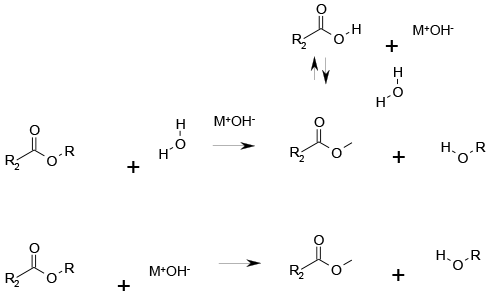
Simple SN2 displacement of alcohol by water/hydroxide or hydroxide if generated in organic solvent ‘naked OH–’.
General comments
If direct hydrolysis with water is kinetically too slow, base can be added to catalyse the reaction. This generates a carboxylate salt which can be isolated or acidified to obtain the free acid. Where water sensitivity or solubility is an issue, a hydroxide soluble in organic solvent can be employed, or a basic phase transfer catalyst. The choice of metal cation will depend on if the metal salt or free acid is the desired product. Generally Na+ or K+ are the best choice. In some cases, weaker bases like carbonate or bicarbonate can be employed.
Key references
Sodium or Potassium hydroxides
The best choice if an inorganic base is needed. K+ salts are usually much more soluble than Na+ salts. Occasionally, metal alkoxides are used with a stoichiometric amount of water to generate ‘naked’ or anhydrous hydroxide.
J. Org. Chem., 1977, 42, 918 procedure for the base-promoted hydrolysis of hindered esters at ambient temperatures
J. Am. Chem. Soc., 1961, 83 , 1743General base catalysis of ester hydrolysis
J. Am. Chem. Soc., 1960, 82, 1778Reactivity of nucleophilic reagents toward esters
MonatsheftefürChemie 2004, 135, 1, 83 Facile hydrolysis of esters with KOH-methanol at ambient temperature
Lithium hydroxide
Lithium hydroxide is not a commonly used reagent for the hydrolysis of esters. The conditions are generally applicable to a wide range of substrates and yield the corresponding carboxylic acid and alcohol in good to excellent yield. Lithium may have selectivity advantages, e.g. can be used in the presence of carbamates. Similar applicability to sodium hydroxide. Frequently water miscible solvents such as alcohols, are added to increase the solubility of the ester. The conditions are not suitable for base-sensitive substrates.
Org. Process Res. Dev. 2012, 16, 1953 2 Kg methyl ester hydrolysis
J. Am. Chem. Soc., 1993, 115, 4497 6-membered lactone opening in synthesis of Lepicidin A
Barium hydroxide
Barium hydroxide is not a commonly used reagent for the hydrolysis of esters on scale. The conditions are generally applicable to a wide range of substrates and yield the corresponding carboxylic acid and alcohol in good to excellent yield. The barium salts of carboxylates can be insoluble, enabling selective monohydrolysis of poly-esters. Barium hydroxide has a similar range of applicability to sodium hydroxide. The conditions are not suitable for base-sensitive substrates.
Tet. Lett., 1977, 18, 4063 ester hydrolysis on g scale
Org. Synth. Coll., 1963, 4, 635. selective hydrolysis of a diacid
Tetrabutylammoniun hydroxide
Tetrabutylammonium hydroxide methods are very similar to other hydroxide based methods in terms of applicability to a wide range of substrates. Yields are generally good to excellent to give the corresponding carboxylic acid and alcohol. One significant advantage of using tetrabutylammonium hydroxide (and other anionic counter ions) is that a biphasic system can be used, with the substrate soluble in the organic phase, that overcomes the issue of poor solubility of the substrate in traditional aqueous alcohol systems. Acetyl-, benzoyl- and pivaloyl-protected alcohols and phenols undergo smooth de-acylation in a two-phase system of powdered NaOH and Bu4NHSO4 in THF or other (aromatics are commonplace). Similar conditions can be used to cleave pivaloyl-protected sugars. Can be a useful approach for base-sensitive molecules, where direct contact with aqueous hydroxide causes unwanted side-reactions.
Synlett, 2003, 991 Removal of Acyl Protecting Groups Using Solid NaOH and a Phase Transfer Catalyst
Relevant scale up example
Sodium and potassium bases
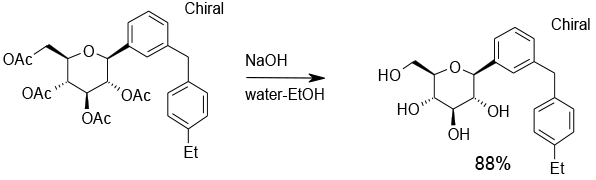
Experimental
70 Kg scale
Org. Process Res. Dev., 2012, 16, 577
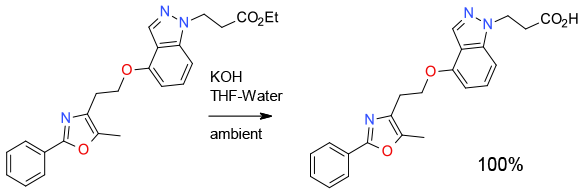
Experimental
100 g scale
Org. Process Res. Dev., 2007, 11, 30
‘Naked’ hydroxide
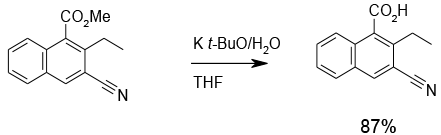
Org. Process Res. & Dev., 2004, 8, 45
Green Review
-
Atom efficiency (by-products Mwt)
High – produces product as a salt and corresponding alcohol. - Safety Concerns
No apparent major operational issues – glass corrosion can be an issue with aqueous solutions >1 M above 40 °C. Care needs to be taken as caustic solutions are corrosive. Soluble barium salts are highly toxic, conversion to the sulfate renders it inert.Strongly basic solutions maybe incompatible with chlorinated solvents (side reactions), though chlorinated solvents are generally discouraged regardless.
- Toxicity and environmental/aquatic impact
Generally minimal once neutralised and diluted. Lithium containing aqueous effluents will be toxic and difficult to treat – Lithium is very toxic to freshwater ecosystems. - Cost, availability & sustainable feedstocks
Cheap and readily available. - Sustainable implications
Very sustainable if aqueous waste can be directly biotreated. Li is currently rated as medium to high risk, and Ba moderate risk for depletion of natural resources.