Biocatalysis
Mechanism + Description
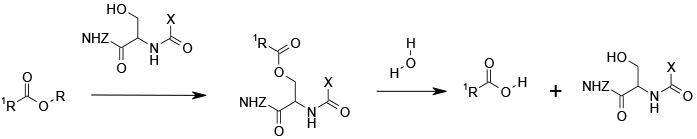
Most hydrolases work via formation of an acyl intermediate bound to serine or cysteine in the active site of the enzyme. Other amino acids in the active site are responsible for the observed rate acceleration. Chiral differentiation is determined by the 3D shape of the active site.
General comments
Many ester groups can be hydrolysed by hydrolytic enzymes –lipases, esterases and proteases. These typically operate under mild conditions and near neutral pH. Some can be used in organic solvents or in aqueous solvent mixtures if water solubility of substrates is an issue. Fundamental attractions of enzyme hydrolysis are selectivity and enantioselectivity (if the hydrolysis is a key transformation in the synthesis of chiral synthons). During the hydrolysis, base may have to be added to maintain an optimum pH range.
Biphasic systems are also viable.
Key references
Enzyme Catalysis in Organic Synthesis (Wiley 3rd Edition) (2011), 1, 251-362 Hydrolysis and formation of carboxylic acid esters
J. Org. Chem., 2005, 70, 3737 Enzymatic Removal of Carboxyl Protecting Groups. 1. cleavage of the t-butyl moiety
Org. Proc. Res. Dev.,2013, 17, 390 Use of bi-phasic systems
J. Org. Chem., 2005, 70, 8730 Enzymatic removal of carboxyl protecting groups. 2. cleavage of the benzyl and methyl moieties
J. Org. Chem., 2007, 72, 782 Enzymatic removal of carboxyl protecting groups. III. fast removal of allyl and chloroethyl esters by bacillus subtilis esterase.
J. Mol. Cata. B: Enzymic, 2000, 10, 357 Biocatalytic resolution of sterically hindered alcohols, carboxylic acids and esters containing fully substituted chiral centers by hydrolytic enzymes
ChemCatChem, 2012, 4, 592 Biocatalyzed regio- and chemo-selective ester cleavage.
Relevant scale up example

Experimental
30 kg scale
Org. Process Res. Dev. 2011, 15, 871
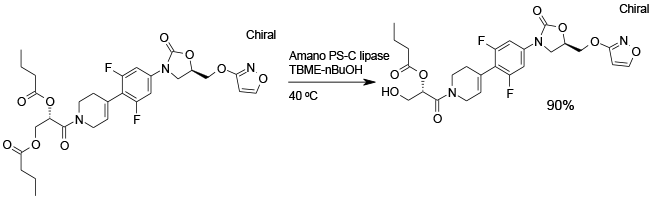
Experimental
5 kg scale
Org. Process Res. Dev., 2006, 10, 644
Green Review
-
Atom efficiency (by-products Mwt)
Excellent with good catalytic activity – only corresponding alcohol as by-product. Though buffers for pH control should not be ignored from an LCA perspective. - Safety Concerns
Generally biocatalytic methods are free of thermal events and can be managed in standard equipment. - Toxicity and environmental/aquatic impact
The biocatalysts are commonly biodegradable and pose minimal environmental hazards. Some of the proteins, especially dusty solids can be sensitizing so appropriate handling should be employed. For use in c-GMP manufacture of API, enzymes from mammalian sources or those fermented using mammalian products should be avoided. Older work using whole cells and biphasic systems often use dialkyl phthalates as the organic phase. These materials are persistent and endocrine disruptors hence should be avoided. - Cost, availability & sustainable feedstocks
Many enzymes are now becoming commercially available in bulk, and many CROs can offer biocatalysis and enzyme development services. In most cases, at the pilot stage, a biocatalysed reaction will cost more than an chemical alternative (fermentation / enzyme supply is very sensitive to economies of scale), but at full scale most biocatalysed processes are greener and cheaper than chemical alternatives. - Sustainable implications
Enzymes are produced from renewable materials and are fully biodegradable back to innocuous natural products (amino acids). The use of modern molecular biology and fermentation technology has greatly reduced the LCI of enzyme manufacture. Maximium sustainability benefits are usually obtained with mutant recombinant enzymes rather than natural enzymes.