Boron and Aluminium reagents, Lewis acids
Mechanism + Description
Activation and cleavage via SN2 reaction.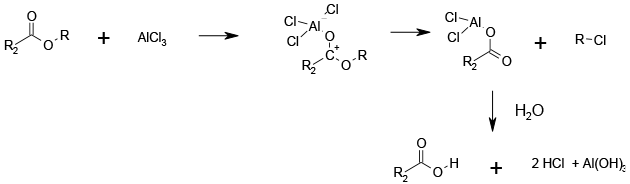
General comments
Lewis acids can be used to cleave esters in two ways.
- After Friedel-Crafts type reactions and quenching, this is essentially acid-catalysed hydrolysis –see acids section.
- Under anhydrous conditions at low or ambient temperatures.
Typically AlCl3 and TiCl4 are used, but others can be employed. Generally weaker Lewis acids need higher temperatures and more forcing conditions. Co-ordination of the metal to the ester increases the leaving group ability of the alkyl group which is cleaved by an SN2 reaction with one of the metal ligands.
Key references
Applied organometal. Chem., 2011, 25, 443 An efficient FeCl3-promoted O-alkyl cleavage of esters to carboxylic acids
Synthetic Communications, 1994, 24, 2179 Mild deprotection of esters with AlCl3–N,N-dimethylaniline
PCT Int. Appl. (1997), WO 9717352 A1 19970515 De-esterification by means of tetrahalides of titanium, tin, and tellurium
Tet. Lett., 1979, 30, 2793 Mild removal of the benzyl ester protecting group with aluminum trichloride
Relevant scale up example
None found
Green Review
-
Atom efficiency (by-products Mwt)
Moderate atom efficiency – generally not all halides are available for reaction. - Safety Concerns
Most Lewis acids are corrosive and react with water/ alcohols, some vigorously. The generation of lower Mwt. alkyl halides may cause off-gassing. - Toxicity and environmental/aquatic impact
Alkyl halides will be generated as by-products. These are alkylating agents and will give rise to positive PGI alerts. There may be issues with discharging aqueous waste with high Al or B content. Hydrolysis of boron-based reagents will lead to boric acid which is a suspected reprotoxic mutagen. Chlorinated solvents are often used and these should be replaced if possible. - Cost, availability & sustainable feedstocks
Reagents are generally readily available at reasonable cost. Neither Al or B are at risk of depletion, but this may not be the case with Lewis acids based on rare metals. - Sustainable implications
Best with Fe, Al or Ti –based Lewis acids.