Hydrogenolysis
Mechanism + Description
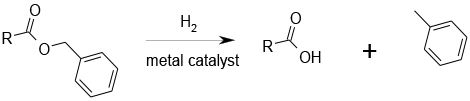
Bonding of the substrate and hydrogen to active sites on the metal leads to cleavage of the ester and generation of the acid and toluene.
General comments
The hydrolysis of esters for preparative purposes using hydrogenation is most commonly used for benzylic esters and structurally related esters. Transfer hydrogenation is a useful alternative, in the presence of 10% palladium on charcoal or palladium black, 1,4‑cyclohexadiene being a very effective hydrogen donor in many reactions, though benzene is an undesired by-product. N-benzyloxycarbonyl, benzyl ester, and tyrosine benzyl ester protecting groups can be efficiently removed within a few hours and to a lesser extent for allylic esters. Selectivity issues can be encountered with isolated double bonds and other functionalities. Low valency sulfur containing molecules can lead to catalyst poisoning as well as P and N lone pairs. Can be scaled with appropriate hydrogenation equipment. Care may be required as mass transfer effects may cause issues. The hydrogenation can be performed in flow reactors.
Key references
Synthesis, 1980, 929 Rapid removal of hydrogenolysable protecting groups under ambient conditions
Perkin Trans. 1, 1977, 490 Transfer hydrogenation; a convenient method for removal of some commonly used protecting groups
J. Org. Chem., 1978, 43, 4194 catalytic transfer hydrogenation with 1,4-cyclohexadiene
Relevant scale up example
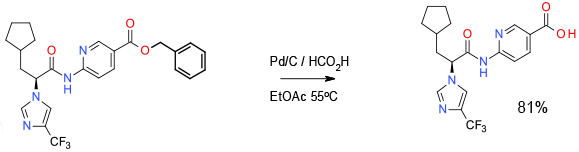
Experimental
42Kg scale
Org. Process Res. Dev., 2012, 16, 1635
Green Review
-
Atom efficiency (by-products Mwt)
With optimized catalytic efficiency, hydrogenolysis is an atom efficient process. - Safety Concerns
Typified by normal hazards around hydrogenation reactions. Catalytic Transfer Hydrogenation (CTH) may avoid the need to handle H2 gas, but these reactions may generate H2 in situ. - Toxicity and environmental/aquatic impact
Main concern is around solubilisation and loss of precious metal/ heavy metal catalysts into waste streams. Most PM/HM levels are tightly regulated. The same applies to potential carry through into the API. Some Ni salts are sensitizers and carcinogens and listed on the EU SVHC list – this is of less concern for metallic hydrogenation catalysts. - Cost, availability & sustainable feedstocks
H2 and H2 obtained via CTH is cheap and non-polluting can be from renewable resources. - Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with efficient recovery and recycling. Pd is the most commonly used precious metal for hydrogenolysis and this is rated at high risk of depletion. No concern for depletion of abundant base metals like Ni.