Metal- Catalysed Cleavage of Allyl Esters
Mechanism + Description
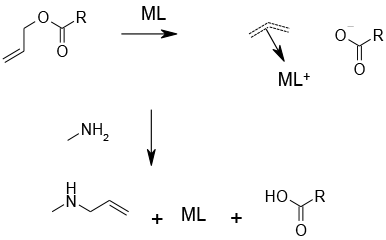
Under the influence of a metal catalyst, the allyl ester binds to a catalytic centre with the expulsion of the anion of the ester. The resulting coordinated π-allyl cation can then react with an acceptor to regenerate the catalyst. The acceptor is normally an amine, but others can be employed.
General comments
Allyl esters can be deprotected under very mild conditions with catalytic amounts of Pd or Ru complexes. This can be useful for polyfunctional molecules where selective deprotection is needed, or molecules that would be decomposed by aqueous hydrolysis.
Key references
Tet. Lett., 1987, 28, 4371 Mild palladium (0)-catalyzed deprotection of allyl esters. A useful application in the synthesis of carbapenems and other β-lactam derivatives.
Angew. Int. Ed.,1984, 23, 71. The allyl group as mildly and selectively removable carboxy-protecting group
J. Org. Chem., 1982, 47, 587 Homogeneous, palladium(0)-catalyzed exchange deprotection of allylic esters
Relevant scale up example
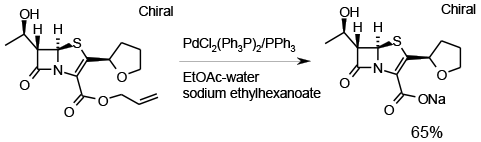
Experimental
10 kg
Org. Process Res. & Dev., 2010, 14, 939.
Green Review
-
Atom efficiency (by-products Mwt)
With an optimized catalyst loading, a reasonable reaction for atom efficiency. The atom efficiency will be maximized by keeping the molecular weight of the allyl cation acceptor as low as feasible. - Safety Concerns
No operational safety issues or hazards apparent with this chemistry. - Toxicity and environmental/aquatic impact
The biggest impact here would be the solvents employed. As with all heavy metal catalysts, contamination of the environment needs to be avoided and levels in the final API need to be controlled. - Cost, availability & sustainable feedstocks
With high catalytic efficiency, this methodology can be an economical way to access complex acids. All metal catalysts have a high LCI from mining and refining and would need to be recovered and recycled. - Sustainable implications
Most precious metals are at risk of depletion (Pd, Pt, Au etc). The use of base metals will be more sustainable.