Metal Iodide/Chloride Salts
Mechanism + Description
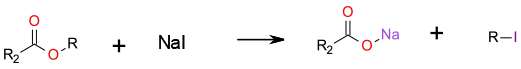
SN2 displacement to generate an alkyl halide and a salt.
General comments
Lithium iodide/chloride in combination with a pyridine derived base can be used to cleave sterically hindered methyl esters in good yield, resulting in the formation of the corresponding carboxylic acid and methyl iodide. The reaction can be extended to other esters. Methyl esters are cleaved faster than ethyl ester, which in turn are faster than secondary alkyls. Interestingly, tert-butyl esters are cleaved rapidly with catalytic lithium iodide (presumably this involves the elimination of hydrogen iodide and 2-methylpropene). The reaction generally requires high temperatures (typically reflux). The reaction is possible under milder conditions if a neighbouring group can coordinate to the lithium ion. An example of which is the cleavage of benzyl, PMB and PNB esters of β-lactams, in non-polar solvents. Magnesium iodide with lithium bromide or chloride have also been used as an alternative iodide source.
Key references
J. Am. Chem. Soc., 2004, 126, 15970 Total synthesis of amphidinolide X
J. Chem. Soc., Chem. Commun., 1984,389 Studies on the synthesis of the antitumour agent CC-1065
Tetrahedron Lett., 1994, 35, 2505 Iodide dealkylation of benzyl, PMB, PNB, and t-Butyl N-acyl amino acid esters via lithium ion coordination
Relevant scale up example
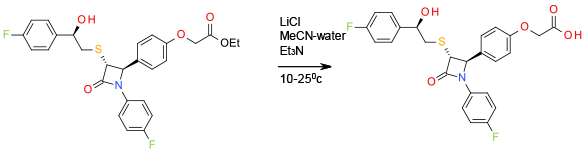
Org. Process Res. Dev., 2012, 16, 586
1.4 Kg
Green Review
-
Atom efficiency (by-products Mwt)
Dependent on salt used. - Safety Concerns
No major issues identified. Low Mwt. alkyl halides may off-gas from reaction and require abatement. - Toxicity and environmental/aquatic impact
Major concerns here would relate more to solvents used than metals. Li and iodide are of concern in fresh water ecosystems. Alkyl halides will be generated as by-products. These are alkylating agents and will give rise to positive PGI alerts. - Cost, availability & sustainable feedstocks
Generally readily available and cheap – though anhydrous metal salts can come at a premium price. Cl and Br are preferred anions over iodide if either option is available. - Sustainable implications
Many of these ester deprotection reactions with metal salts are conducted in dipolar aprotic solvents, which should be replaced if possible. Use of iodide – incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion, although it is possible to recover iodide from waste materials. Li and Mg are rated at moderate risk of depletion.