Water
Mechanism + Description
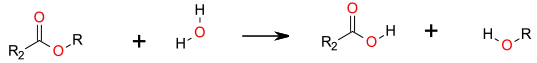
Simple SN2 displacement of alcohol.
General comments
Many activated esters e.g. trifluoroethyl, benzoyl, trifluoroacetate etc can be hydrolysed with water or water-solvent mixtures alone or added to an organic solvent if needed for solubility. This should always be tried as a first line of investigation.
Key references
J.Am.Chem.Soc., 1958, 80, 2985 – Hydrolysis of t-Alkyl Trifluoroacetates
Relevant scale up example
None found
Green Review
-
Atom efficiency (by-products Mwt)
High – produces product and corresponding alcohol, negligible waste and water can be used as the solvent. - Safety Concerns
None prominent. Higher temperatures may be required to push reaction to completion (stability of SM/product). - Toxicity and environmental/aquatic impact
Minimal. - Cost, availability & sustainable feedstocks
Cheap and readily available. - Sustainable implications
Sustainable. Aqueous waste can potentially be directly bio-treated.