Peptide Synthesis
The inclusion of an article in this document does not give any indication of safety or operability. Anyone wishing to use any reaction or reagent must consult and follow their internal chemical safety and hazard procedures and local laws regarding handling chemicals
*CMR = Carcinogenic, mutagenic, reprotoxic
Users need to ensure that reagents/chemicals are available at the right scale/cost for individual requirements. For legal reasons, recommendations for suppliers cannot be made in these guides. Many free to use and fee for service search engines are available for chemical sourcing.
General Overview
Peptides have long been an important class of pharmaceuticals. Historically targeted towards high-potency, low-patient volume disease areas such as oncology, many peptide-based therapies are now launched or in development for high-volume diseases such as cardiovascular and metabolic therapy areas. The demands for higher volumes of peptides and more complex molecules, (e.g., high molecular weight, cyclic, stapled and those conjugated with other chemical classes), and a move towards more sustainable pharmaceutical manufacturing, has led to a number of advances in large-scale peptide synthesis. Traditionally, peptide manufacture has been associated with large excesses of reagents and solvents, with some commercial peptides generating 34 tons of waste and 118 tons CO2 eq. per kg of API.
A variety of methods are used to access peptides:
- Extraction – for natural products
- Recombinant technologies – Recombinant synthesis is particularly of use for very large amounts of long peptides composed of natural amino acids. There are molecular biology techniques that allow the inclusion of a non-canonical or unnatural amnio acid.
- Biocatalysis – to ligate larger peptide fragments using isolated enzymes.
- Traditional solution chemistry liquid phase peptide synthesis (LPPS) – short peptide length, low volumes
- Solid phase peptide synthesis (SPPS) – uses large amounts of amino acid synthons, but a much lower burden in work-up and purification
- Hybrid approaches, e.g., solid-solid or liquid-solid to access more convergent rather than linear routes – generally chosen for peptides that are greater than ~ 25 amino acids in length, and higher commercial requirements of 10-200kg
This guide focuses on emerging technologies and strategies for the greener synthesis of peptides. The basic bond forming chemistry in peptide synthesis is repeated amide bond formation and deprotection. For guidance on specific amide bond forming reactions, see the amide bond formation reagent guide (coming soon).
General Literature Reviews on Greener Peptide Synthesis
Green Criteria for peptide synthesis
- Large molar excesses of reagents should be avoided if possible or minimized while maintaining yield and purity requirements for the final product.
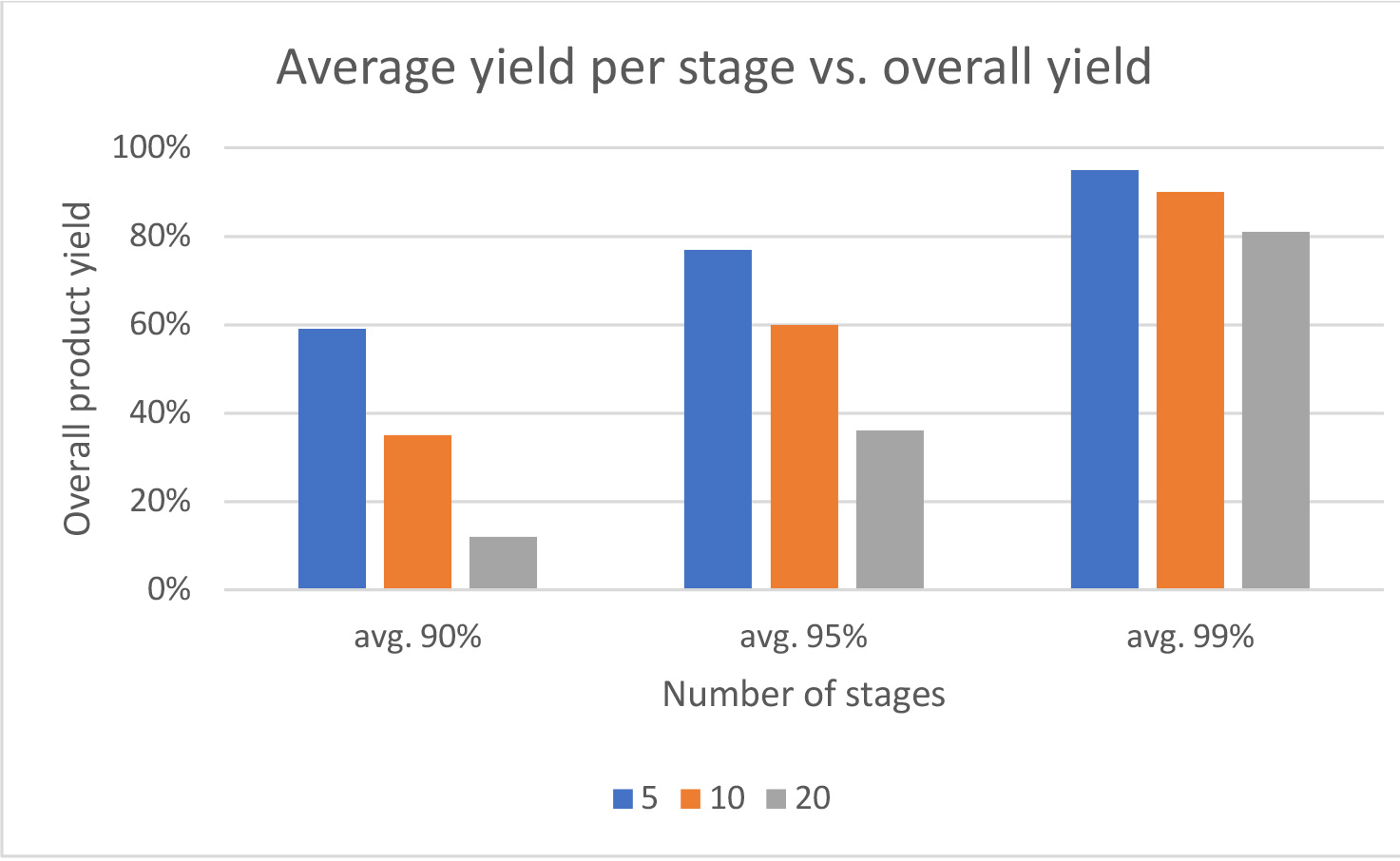
- Yields for each coupling and deprotection step should be maximized, especially in long linear sequences. The table below highlights the effects on overall synthesis efficiency of lower yields per stage – 90,95 and 99% for a 5,10 and 20 mer peptide.
- For longer sequences, convergent sequences should be considered.
- In solid phase or hybrid modes, continuous or flow processing maybe beneficial when compared to traditional batch processes.
- High-impact solvents like diethyl ether, dichloroethane, DMF, DMAC, NMP should be avoided if possible. For alternatives, see guide.
- Solvent use minimized and solvents recovered and reused where feasible.
- SPPS may be more efficient if operated in flow rather than batch.
- In SPPS, polymer resins are used once and then disposed. Typically, these polymers have a high LCI burden and replacing traditional SPPS with tagging the peptide and using hydrophobic/hydrophilic solvent phase changes or solvent resistant nanofiltration may be more sustainable alternatives.
- Work-up/DSP should be optimized for efficiency; if possible, avoid resource and energy intensive downstream processing.