Molecular Solvents – Replacements for DMF, DMAC, NMP
A number of publications have described alternatives to DMF in the synthesis of a range of peptides across varying degrees of scale. Looking at the available data—and many authors also make this point—it is clear that there is no single universal solvent that can replace DMF in SPPS, but viable substitution maybe available and this is often a mixture. Mixtures or solvent composition may need to be changed for optimum results in the coupling and deprotection steps.
The optimum substitution will depend on each resin and peptide sequence will need to be evaluated by screening each individual case prior to deciding on common problems include undesirable resin swelling, changes in bed volumes if solvent composition is changed, insolubility of AA synthons and lower yields and purities of the product peptide. A number of binary mixtures have been shown to mitigate some common side-reactions in SPPS.
A number of greener solvents have been used to anchor the first AA residue to the solid but may be less useful for subsequent coupling steps.
Some suggested replacements for DMF:
- Cyrene™ (dihydrolevoglucosenone), sulfolane or anisole with dimethyl carbonate or diethyl carbonate
- N-butyl-2-pyrrolidinone (NBP) – non CMR replacement for NMP. In some instances, this solvent has been used but issues noted include its cost is too high for large-scale manufacture and neat NBP being too viscous in SPPS. In some cases, NBP mixtures have been employed. Other higher molecular weight N-alkylpyrrolidones such as N-octyl-2-pyrrolidone (NOP), N-cyclohexyl-2-pyrrolidone (NCP) and N-benzyl-2-pyrrolidone (NBnP) have been used in SPPS. Regarding cost and availability, NOP is also known as Surfadone™ and is widely used in a range of industries, and the cost is comparable to more widely used common solvents (about $1–2 per kg). Attention to the quality of the NOP would be needed if used in c-GMP synthesis.
- NBP and 2-MeTHF mixtures
- NBP and 1,3-dioxolane (DOL) mixtures
- NOP and dimethyl carbonate mixtures (MeO2CO) reduces viscosity of NOP
- N-formyl morpholine (NFM) and DOL mixtures
- DMSO and EtOAc mixtures – can give good results in chemistry steps and can also be recycled thus lower effect PMI for the overall process.
- Propylene carbonate, a green polar aprotic solvent which can be used to replace dichloromethane and DMF in both solution and SPPS.
- γ valrolactone (GVL) has been used for amide bond formation in SPPS. In common with ester solvents, the use of GVL can lead to undesired capping of the peptide N-terminus by amide formation.
Rather than totally replace DMF, some adopt the strategy of retaining DMF for the amide coupling step, then replacing DMF for the deprotection and/or the wash/purification stages. Some suggested replacements for DMF in washing phase:
- DMSO in combination with DOL, 2-Me-THF or EtOAc are suitable for efficient Fmoc-removals
- EtOAc-MeCN mixtures
- EtOAc
- 2% DMSO EtOAc
- NBP in MeCN or EtOAc
- DMPU in EtOAc
- NOTE: Solvents like NFM and DMPU may end up classified as carcinogenic, mutagenic, or toxic for reproduction (CMR substances) alongside DMF. DMPU hazard class label H361f – suspected of damaging fertility.
General Greener Solvents
NBP & NOP
Carbonates
Cyrene
GVL
Deep eutectics (DES) and ionic liquids
A number of publications have optimized solvent mixtures looking at polarity and ability to swell the resins usually used in SPPS.
The overall performance of binary solvent mixtures in SPPS correlated to the polarity and viscosity of the solvents and solvent mixtures benchmarked to the widely used DMF.
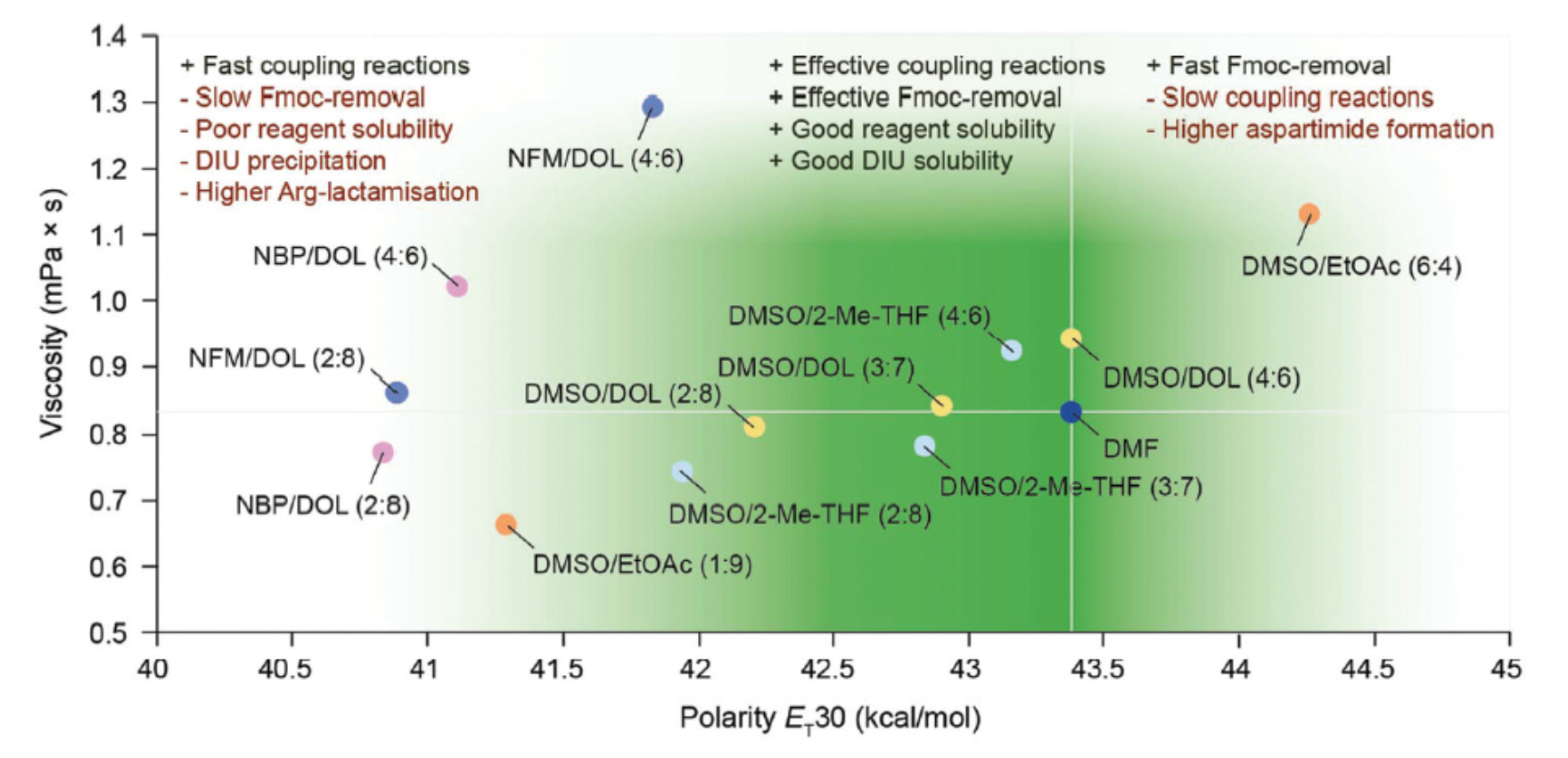
Reproduced with permission from the Royal Society of Chemistry
Ran, Y.; Byrne, F.; Ingram, I. D. V.; North, M. Resin Swelling in Mixed Solvents Analysed using Hansen Solubility Parameter Space. Chem. Eur. J. 2019, 25, 4951–4964.
Amadi-Kamalu, C.; Clarke, H.; McRobie, M.; Mortimer, J.; North, M.; Ran, Y.; Routledge, A.; Sibbald, D.; Tickias, M.; Tse, K.; Willway, H. Investigation of Parameters that Affect Resin Swelling in Green Solvents. ChemistryOpen 2020, 9, 431–441.
Suggested idea solvent parameters:
- Solvent melting point ≤ 10 °C
- Solvent viscosity < 4 mPa s
- Reagents and by-products solubility ≥ 0.25 M
- Starting resin swelling 4–7 mL g−1
- Coupling time ≤ 1 hour
- Fmoc-removal time ≤ 30 min