More Efficient LPPS
A range of reagents have been developed (some now being scaled/used on scale) using group-assisted purification (GAP) chemistry. This technology allows the use of a tag molecule to anchor the peptide. Often several growing peptide chains can be attached to a single tag molecule. The resulting macromolecule/tag complex imparts properties that allow for much faster and more efficient washing/purification compared to SPPS.
GAP LPPS produced intermediates that are:
- Soluble in polar organic solvents; insoluble in non-polar solvents; can be purified by precipitation.
- Soluble in organic solvents but insoluble in water allowing water washing to remove excess reagents.
- Of a suitable molecular size that solvent-resistant nanofiltration can be used to purify after each stage. The peptide macromolecule retained in the retentate and excess reagents and soluble impurities passing in the permeate.
At the end of the synthesis, the tag molecule is typically removed using acid deprotection.
- Uses widely available amino acid derivatives that are used in classical Fmoc/tBu- SPPS
- Reagents can be used in lower excess when compared to classical SPPPS
- Does not require isolation of intermediates
- Better in-process control (IPC) is readily achieved
- Overall solvent consumption is significantly reduced, up to 75%
- Use of CMR solvents can be avoided or reduced.
Examples of GAP tag molecules
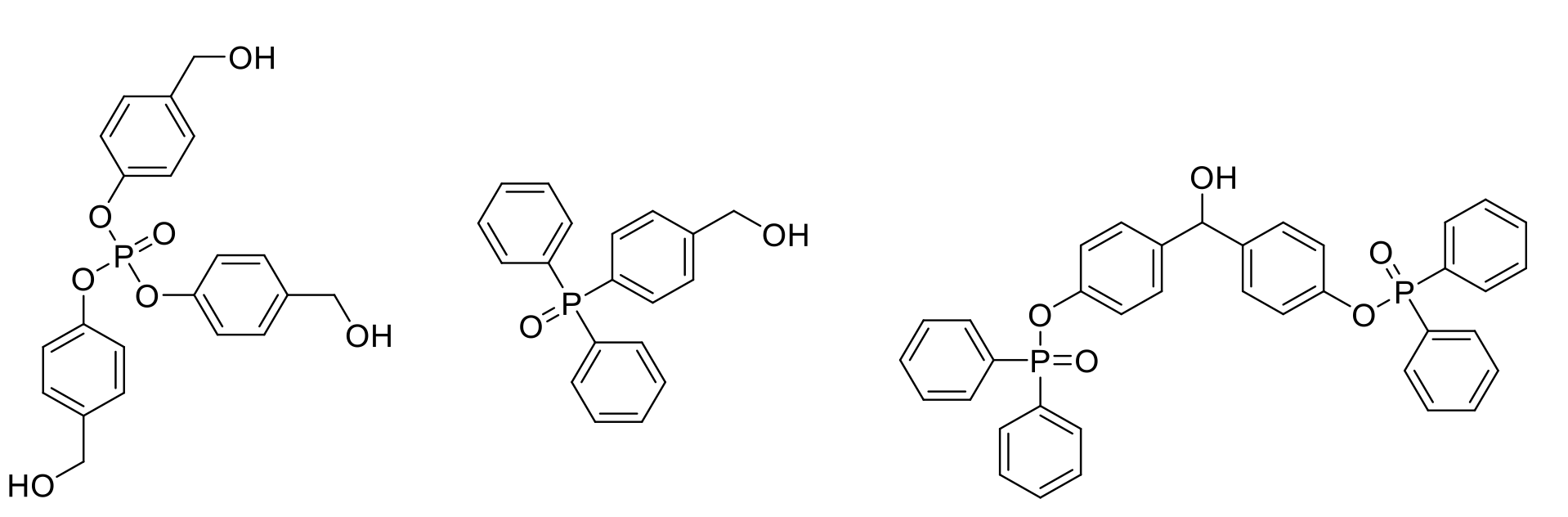
AJIPHASE
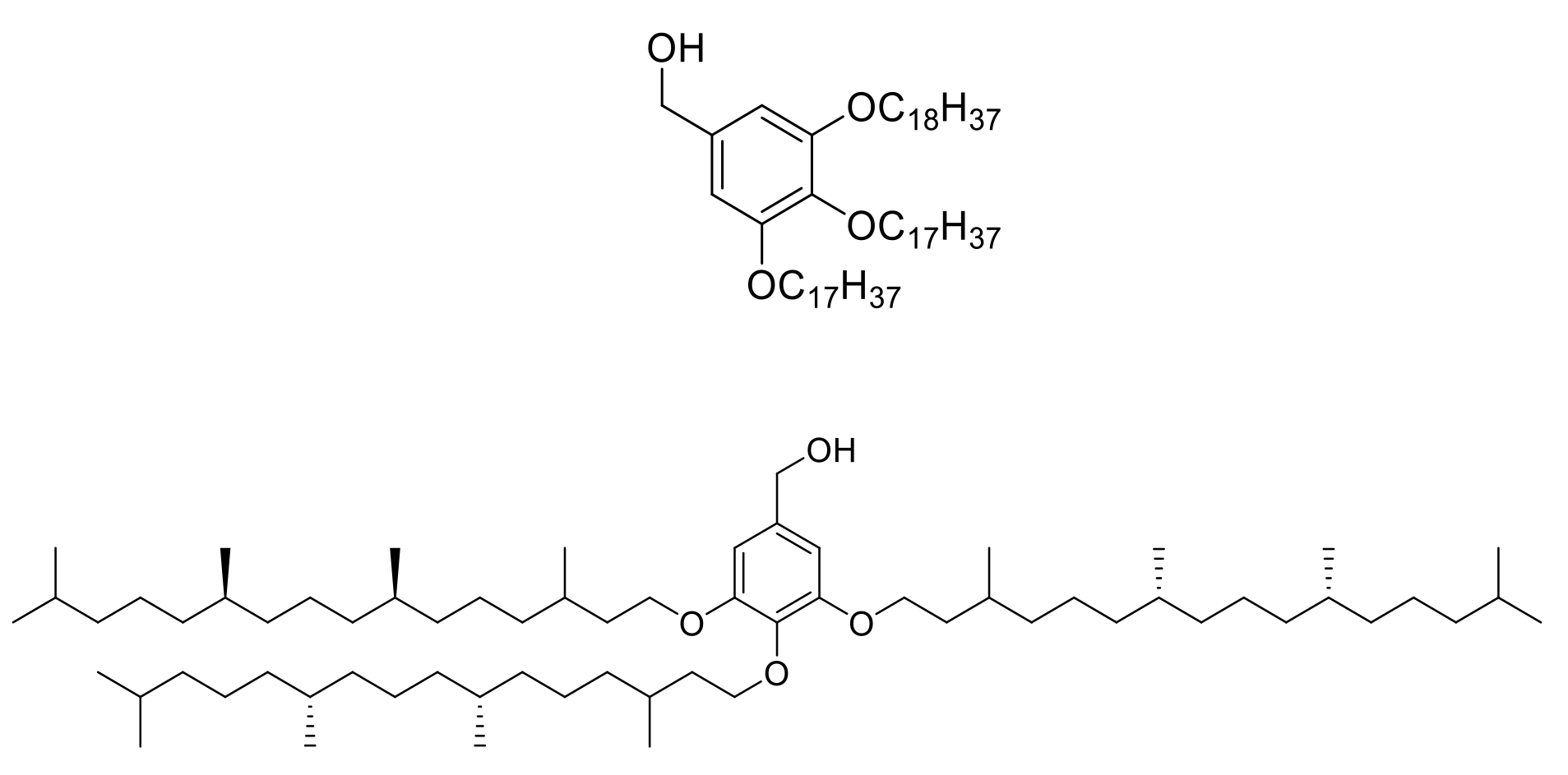
Based on phytol
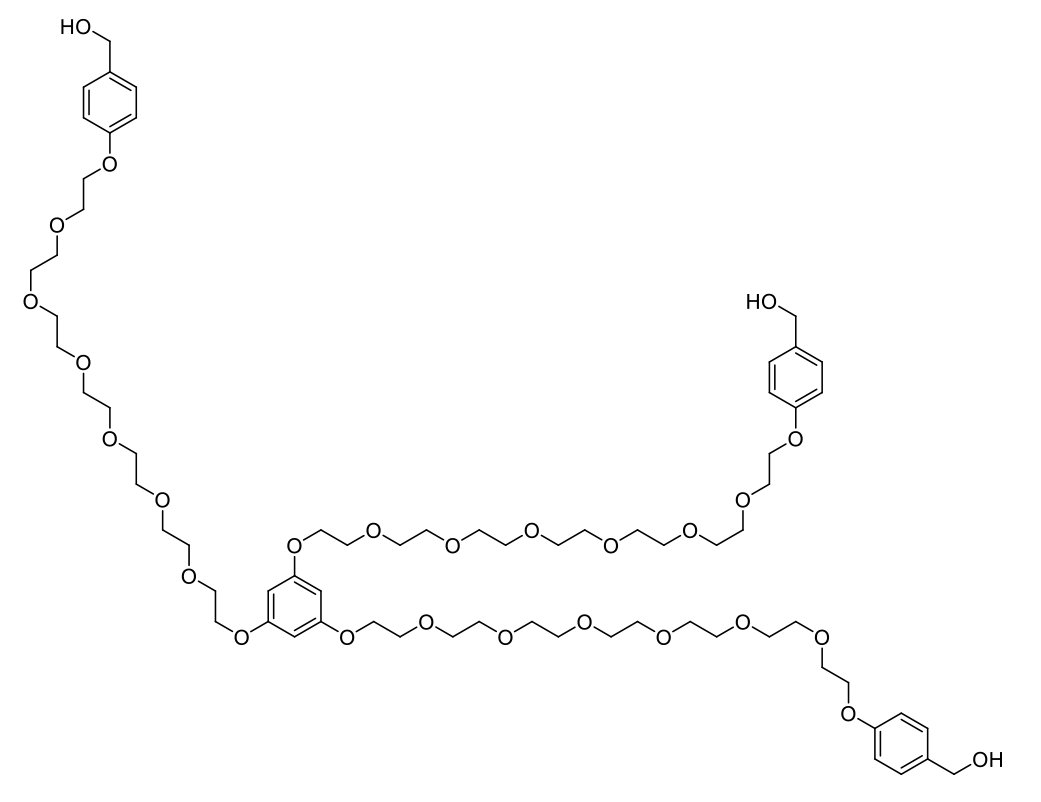
Membrane enhanced peptide synthesis (PEPSTAR) linker
Chen, W.; Sharifzadeh, M.; Shah, N.; Livingston, A. G. Iterative peptide synthesis in membrane cascades: Untangling operational decisions. Comput. Chem. Eng. 2018, 115, 275–285.