Alkylation with Functionalised Alkanes
Mechanism + Description
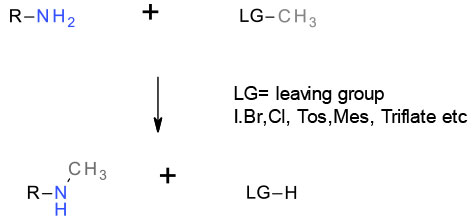
Normally SN2 generating conjugate acid of the leaving group or a salt if a base is added to reduce acidity and reduce protonation of the nucleophilic amine. Clean SN2 reaction leads to inversion of a stereo centre at a reacting carbon. Electrophiles capable of forming stable Carbocations can react via an SN1 mechanism.
General comments
Alkyl halides are very commonly used to construct amines – reactivity follows RI > RBr > RCl >>RF.
Due to low reactivity, alkyl fluorides are never used. Often the most reactive iodides are formed in situ by adding I- – Finkelstein catalysis. Often stoichiometric amounts of salts like KI are used, but the reaction can be catalytic in iodide (10 mol% or less). Sulfonates are also widely used – usually mesylate or tosylate, but other leaving groups like nosylate or brosylate are occasionally found along with very reactive fluorosulfonates like triflate. Sulfonates are sometimes formed ‘in situ’ from alcohols and not isolated. Reactivity of sulfonates is similar or better than the more reactive alkyl halides.
Alkylation of amines by alkyl halides/ sulfonate esters is normally via SN2 displacement. The reaction is of wide utility and generally provides high yields and clean inversion of stereochemistry. For halides of sulfonates that form cations, the SN1 pathway may predominate. Where the alkyl halide/sulfonate or amine are hindered, elimination may be a side reaction.
Key references
Comprehensive Organic Transformations, Wiley, NY, 2nd edition, pp. 779-784, 789-792 review article
Tetrahedron 2001, 57, 7785-7811 synthesis of secondary amines
A comparison of several modern alkylating agents
Cyclic sulfates can be used as electrophiles – these are analogous to epoxides and carbonates but show much higher reactivity.
These can be prepared via oxidation of the corresponding cyclic sulfites. The amino sulfate products are usually hydrolysed to amino alcohols.
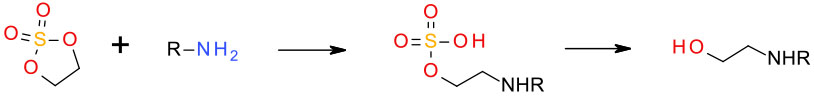
J. Am. Chem. Soc., 1988, 110 (22), 7538–7539 Vicinal diol cyclic sulfates (like epoxides only more reactive)
J. Org. Chem., 1990, 55, 1211–1217 Cyclic sulfates: useful substrates for selective nucleophilic substitution
Relevant scale up example
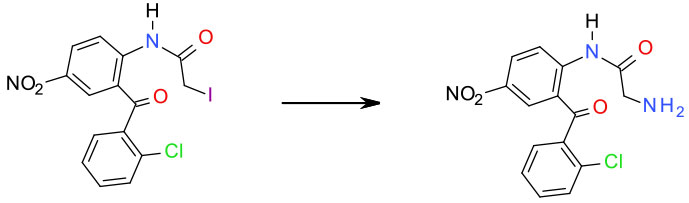
Experimental
EtOAc NH3 gas 3 Kg scale
Org. Process Res. Dev. 1997, 1, 268-272
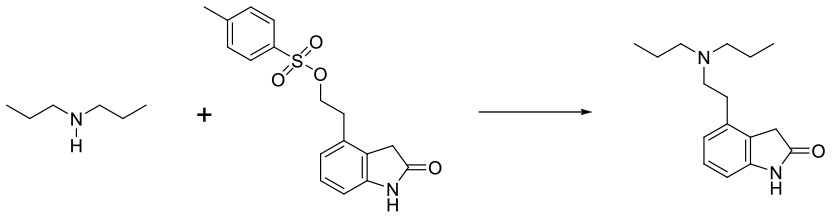
Experimental
H20/ 10 eq HNiPr2 65 Kg scale
Org. Process Res. Dev. 1998, 2, 3-9
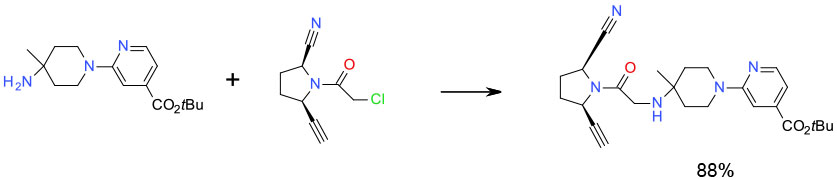
Experimental
30 Kg scale
NMP/ KI cat/ milled K3PO4
Org. Process Res. Dev. 2009, 13, 1145–1155
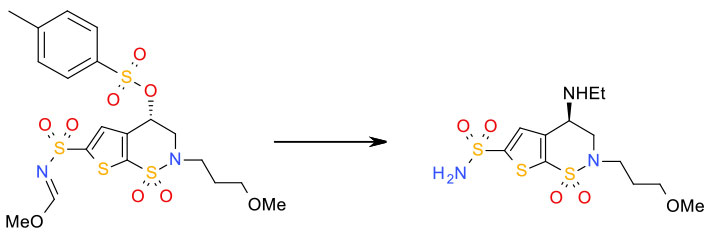
Experimental
THF/ Et3N/H2NEt aq / 500 g scale
Org. Process Res. Dev. 1999, 3, 114-120
Green Review
-
Atom efficiency (by-products Mwt)
This comes down to the leaving group Cl < Br < I. Sulfates and sulfonic esters produce as high or higher Mwt. By-products than Cl or Br. - Safety Concerns
No major safety concerns with the operational aspects of alkylation. All alkylating agents will have positive PGI alerts and are know or suspect carcinogens so should be handled with appropriate caution. Use of alkylating agents towards the end of a synthesis may give a requirement to measure very low levels of alkylating agent in the API. See Org. Process Res. Dev. 2013, 17, 221−230 The level of concern rises with reactivity of the electrophile. Low Mwt sulfonates can also have acute toxicity e.g. methyl fluorosulfonate (‘magic methyl’). - Toxicity and environmental/aquatic impact
This is really down to the ability of the reagent to biodegrade and it’s reactivity. Some are very toxic e.g. benzyl halides. Higher molecular weight, lipophilic materials may show bioaccumulation. Sulfates and sulfonate esters are generally rapidly hydrolyzed and environmental concerns reflect those of the alcohol. Fluorosulfonated anions can be persistent in the environment. High concentrations of iodide are harmful to freshwater ecosystems. Use of dipolar aprotic solvents should be avoided if possible. - Cost, availability & sustainable feedstocks
Many alkyl halides are readily available and cheap, or can be prepared from the corresponding alcohols. Sulfates and sulfonate esters can be prepared in situ just before use to minimize handling and the potential of exposure. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. A high LCI reagent, although it is possible to recover iodide from organoiodine waste materials.