Ammonia Substitutes
Mechanism + Description
If primary amines are the key product, NH3 is the greenest amine source.
However, issues can arise with the use of ammonia – poor reactivity, high volatility, and multiple alkylation. Depending on the target and alkylating agent, NH3 in solution or as a gas can be used, but often a surrogate is chosen and later converted to RNH2
General comments
A number of synthons equivalent to the use of NH3 have been reported. Generally, the addition of Acyl or sulfonyl groups lowers reactivity, and the fully deprotonated version may be required, these are listed below.
Azide and Azide donors – TMSN3 NaN3 or Ph3P(O)N3
Imides like Phthalimide is an often used synthon – deprotected with hydrazine , ethylene diamine or 40% MeNH2 aq. Succinamide is more atom efficient. Better still is diformamide, which can be cleaved to the primary amine under acidic conditions
hexamethylenetetramine
Other NH3 surrogates that are used but show poor atom economy are tritylamine and dibenzylamine. Hexamethyldisilazide and salts are readily deprotected, but again not very atom efficient.
Boc analogs: BOC2NH, Boc-NH-OCbz, Boc-NH-O-Ns, TMS(CH2)2NHBoc, BocNHCO2CH2CF3, BocNHP(O)(OEt), Boc-NHN=CMe
Mg3N2 – magnesium nitride, see safety concerns in next section.
Relevant scale up example
(see also Mitsunobu rxn, and references Section 1)
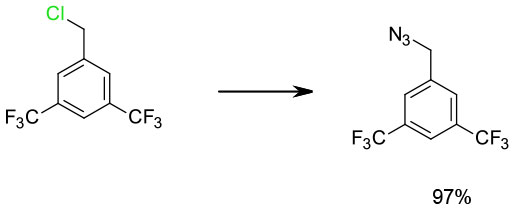
Experimental
Isopropyl acetate/H2O/NaN3/ nBu4NBr
Alternatively rxn can be performed in flow mode in DMSO-H2O 9:1V/V
Org. Process Res. Dev. 2009, 13, 152–160
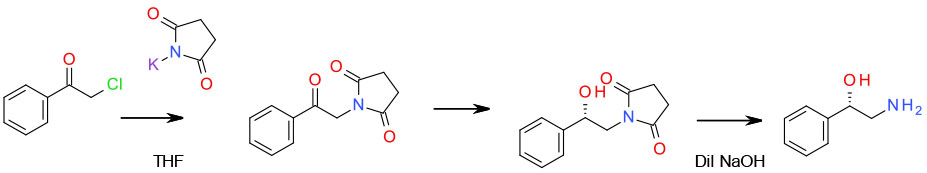
Use of succinimide as an
ammonia substitute
Org. Process Res. Dev. 2006, 10, 893-898
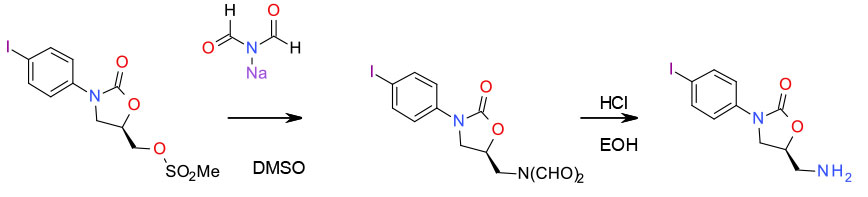
One-Pot Process for the Amination of Oxazolidinyl-methyl Mesylate by Sodium Diformylamide
Org. Process Res. Dev. 2007, 11, 739-741
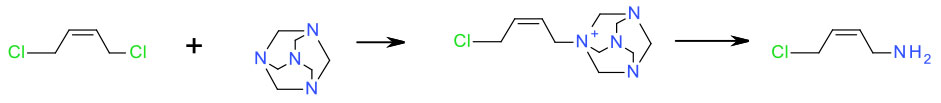
Experimental
- DCM/ hexamethylenetetramine
50 Kg scale - MeOH/HCl
Org. Process Res. Dev. 2009, 13, 638–640
Green Review
-
Atom efficiency (by-products Mwt)
NH3 is the most atom efficient reagent – if this cannot be used, then choosing the lowest Mwt alternative will maximize atom efficiency. Overall, the effect of any deprotection steps and reagents also needs to be considered. The use of protecting groups to avoid over-alkylation will decease atom efficiency. - Safety Concerns
Specific to the reagents being used: two require highlighting – Azide and Azide donors – NaN3 or Ph3P(O)N3 – main hazard is the thermal stability of organic azides and the potential to generate HN3 gas and the resulting explosion hazard. Mg3N2 – magnesium nitride –has been touted as an NH3 substitute (Org. Lett. 2008, 10, 3621) This reagent should be handled and scaled with great care as explosions have been reported Org. Process Res. Dev. 2009, 13, 1388–1394 - Toxicity and environmental/aquatic impact
Ammonia and reagents that rapidly generate ammonia are harmful to freshwater ecosystems. (TMS)2O, a by-product from N-TMS reagents can bio-accumulate. BOC reagents will generate isobutylene and other gases upon deprotection. - Cost, availability & sustainable feedstocks
Ammonia and simple substitutes like succinimide, phthalimide are cheap and readily available. Succinimide can be made from renewable resources. - Sustainable implications
Ammonia should be used whenever feasible – other ammonia substitutes and the use of protecting groups to avoid over alkylation will inevitably lead to a higher environmental burden.