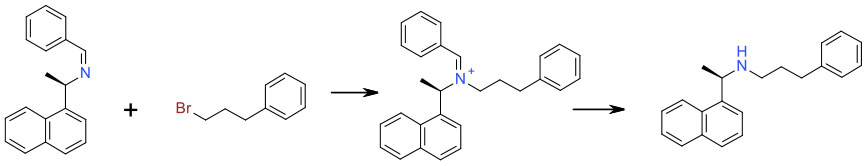
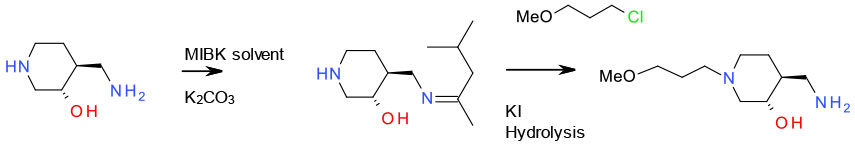
Avoiding Over-alkylation
Mechanism + Description
In order to avoid issues with mixtures resulting from over- alkylation, protecting groups or ammonia substitutes can be used when primary or secondary amines are the target products.
General comments
When reactive nuclophiles like amines are reacted with electrophiles like alkyl halide/sulfonates or epoxides, over alkylation is always problematic. This tends to be most acute when the desired products are primary or secondary amines. From a green aspect, this is best approached through careful optimisation of stoichiometry, solvent and base, dilution and possibly flow chemistry. Large excess of amine or alkylating agent are detrimental to atom economy and the presence of excess mutagenic reagents (PGI’s) can cause issues especially close to the end of a synthetic route. An amine with multiple nitrogen groups may also give rise to selectivity issues requiring protection / deprotection.
Relevant scale up example
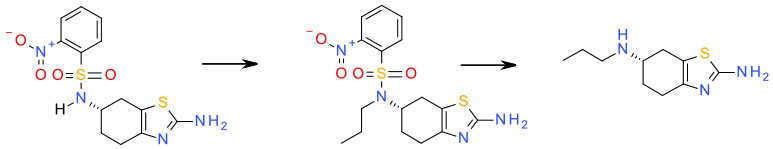
Experimental
1) MeCN/K2CO3/PrBr
2) EtOH/LiOH/thioglycolic acid
Org. Process Res. Dev. 2010, 14, 1125–1129