Carbonates
Mechanism + Description
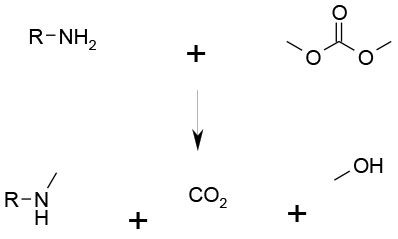
SN2 reaction leading to product, CO2 and an alcohol
General comments
Carbonates have several positive aspects as alkylating agents, although currently limited to a small range of alkyl groups in commercially available carbonates. Often used for alkylation of anilines and related compounds. Benefits are:
- Non-toxic
- Innocuous leaving group
- Can be prepared from CO2 and alcohol
This needs to be balanced against much lower reactivity which is poor compared to alkyl halides and sulfonates due to low leaving group reactivity. There can also be competing reaction at the carbonyl centre. However, reactivity can be addressed using catalysts like DABCO or ionic liquids, or working at higher temperature. A large excess of carbonate is often employed which gives rise to poor mass efficiency unless recovered.
Key references
Chem. Rev. 1996, 96, 951-976 review on carbonate chemistry
Acc. Chem. Res. 2002, 706 The Chemistry of Dimethyl Carbonate
J. Mol. Catal. A. Chem. 2005, 226, 49-56 Selective synthesis of N,N-dimethyl aniline derivatives using dimethyl carbonate as a methylating agent and onium salt as a catalyst
Green Chemistry 2009, 11(8), 1161-1172 The reaction of glycerol carbonate with primary aromatic amines in the presence of Y- and X-faujasites
Relevant scale up example
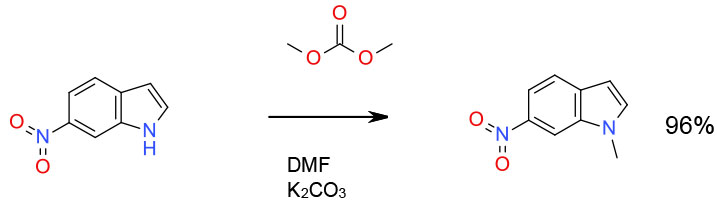
Experimental
300 gallon scale
Org. Process Res. Dev., 2001, 5 (6), 604–608
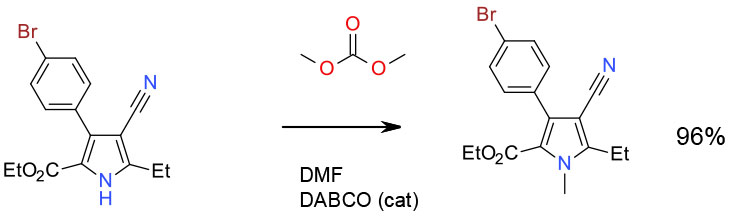
Experimental
Gram scale
Org. Process Res. Dev., 2009, 13 1199–1201
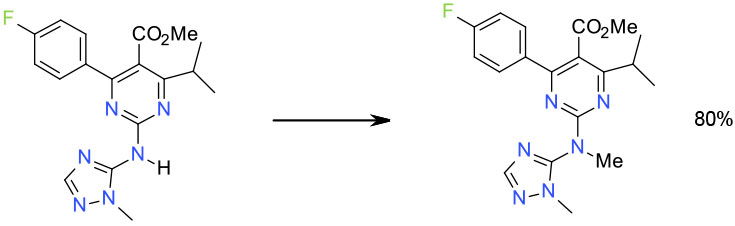
Experimental
20 Kg scale
Toluene/ (MeO)2CO /DABCO
Org. Process Res. Dev. 2010, 14, 441–458
Green Review
-
Atom efficiency (by-products Mwt)
Alkylation generates one mole of CO2 and one mole of the corresponding alcohol, so atom efficiency is moderate. By-products are beguine. - Safety Concerns
No major operational hazards apparent. The use of carbonates often means working at higher temperature to other more reactive alkylating agents. Gas can be evolved. - Toxicity and environmental/aquatic impact
Generally good- hydrolysis will generate CO2 and low Mwt. alcohols. Toxicity and impact mirror that of the corresponding alcohol. - Cost, availability & sustainable feedstocks
Many carbonates are available cheaply and in bulk and Carbonates derived from simple alcohols can be manufactured from renewables. - Sustainable implications
Use of carbonates can remove hazards associated with other reactive alkylating reagents, and can be bio-derived.