Catalytic Asymmetric Allylic Amination
Mechanism + Description
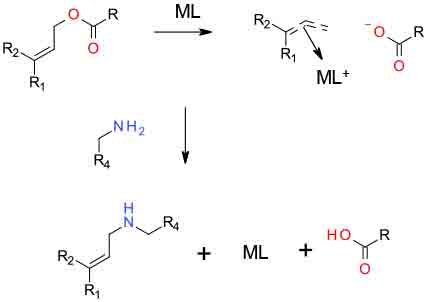
Under the influence of a metal catalyst, a suitably substituted allyic reagent binds to a catalytic centre with the expulsion of a leaving group e.g. acetate. The resulting coordinated π- allyl cation can then react with a nuclophile to generate the allyic amine and regenerate the metal catalyst.
General comments
A reaction of wide utility but scope limited to allylic substrates with leaving groups able to form metal coordinated π- allyl cations. Leaving groups include carbonates, phosphate and acetate. Notably, Hartwig has achieved high ee with use of amines and allylic alcohols with only OH as the leaving group. These coordinated pi allyl cations can react with a variety of reagents, especially nitrogen nucleophiles of a wide scope, including alkyl amines, heterocyclic amines, aromatic amines, azide, sulfonamide, imide, guanidines, and sulfamic acid. The reaction is readily amenable to scale-up.
Key references
Org. Process Res. Dev., 2012, 16, 185–194 Pd- and Mo-Catalyzed Asymmetric Allylic Alkylation
Chem. Rev. 1996, 96, 395-422 Asymmetric Transition Metal-Catalyzed Allylic Alkylations
Chem. Rev. 2003, 102, 2921-2943 Asymmetric Transition-Metal-Catalyzed Allylic Alkylations: Applications in Total Synthesis
Org. Lett., 2003, 5 1809–1812 Asymmetric Rearrangement of Prochiral Allylic Alcohols to Chiral Allylic Amines
J. Org. Chem., 2005, 70 (2), pp 648–657 Asymmetric Synthesis of Chiral Allylic Amines
J. Am. Chem. Soc. 2009, 131, 4200-4201 Palladium-Catalyzed Allylic Amination Using Aqueous Ammonia
Org. Lett. 2008, 12, 2425-242 Synthesis of Dehydro-β-amino esters via Highly Regioselective Amination of Allylic Carbonates
J. Am. Chem. Soc. 2007, 129, 7508-7509 Iridium-Catalyzed, Asymmetric Amination of Allylic Alcohols Activated by Lewis Acids
Angew. Chem, Int Ed. 2007, 46, 3139-4143 Iridium-Catalyzed Synthesis of Primary Allylic Amines from Allylic Alcohols: Sulfamic Acid as Ammonia Equivalent
J. Org. Chem. 2009, 74, 305-311 Modular Phosphite–Oxazoline/Oxazine Ligand Library for Asymmetric Pd-Catalyzed Allylic Substitution Reactions: Scope and Limitations—Origin of Enantioselectivity
Relevant scale up example

Experimental
Gram scale
Org. Process Res. Dev., 2003, 7, 432–435
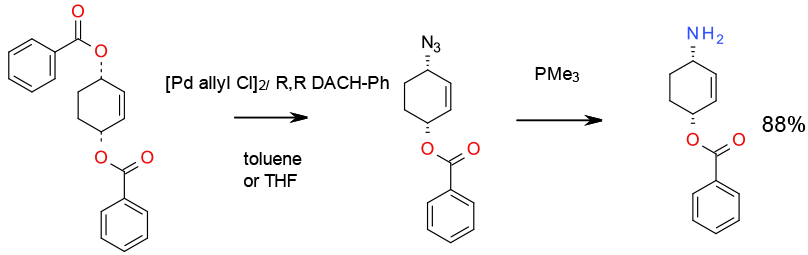
Experimental
5.5 Kg scale
Org. Process Res. Dev., 2012, 16, 2051–2057
Green Review
-
Atom efficiency (by-products Mwt)
With an optimized catalyst loading, a reasonable reaction for atom efficiency generating a salt of the leaving group. Obviously, leaving groups with as low Mwt. and with low environment impact as possible should be used – acetate is preferable to benzoate etc. - Safety Concerns
No operational safety issues or hazards apparent with this chemistry - Toxicity and environmental/aquatic impact
The biggest impact here would be the solvents employed. As with all heavy metal catalysts, contamination of the environment needs to be avoided and levels in the final API need to be controlled - Cost, availability & sustainable feedstocks
With high catalytic efficiency, this methodology can be an economical way to access complex chiral amines. All metal catalysts have a high LCI from mining and refining. Ideally, they would be recovered and recycled. - Sustainable implications
Most precious metals are at risk of depletion – Mo (medium risk of depletion) is preferred over Pd/Ir/Rh (high risk of depletion)