Epoxides and Azridines
Mechanism + Description
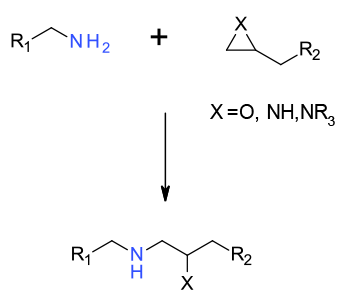
SN2 reaction between amine and epoxide/azridine as the electrophile
General comments
Epoxides are versatile synthons and reaction with amines lead to amino alcohols and related compounds. Epoxides can be employed or isolated or formed in situ. Since many enantiomerically pure epoxides can be obtained, this route is often a good source of highly functionalised chiral materials. Regio- and enantioselectivity issues can often be controlled by choice of reaction conditions, solvents , temperature and additives. Whilst less commonly used, azridines can be used in analogous reactions to epoxides.
Key references
J. Am. Chem. Soc. 2006, 128, 6312-6313 De Novo Synthesis of Tamiflu via a Catalytic Asymmetric Ring-Opening of meso-Aziridines with TMSN3
Org. Lett., 2005, 7 4593–4595 Catalytic Asymmetric Ring Opening of meso-Epoxides with Aromatic Amines in Water
Org. Lett., 2005, 3649–3651 Highly Chemoselective Addition of Amines to Epoxides in Water
Tetrahedron 2001, 57, 7785 Synthesis of secondary amines
Current Organic Chemistry 2005, 9(1), 1-29 Asymmetric ring opening of epoxides
Relevant scale up example
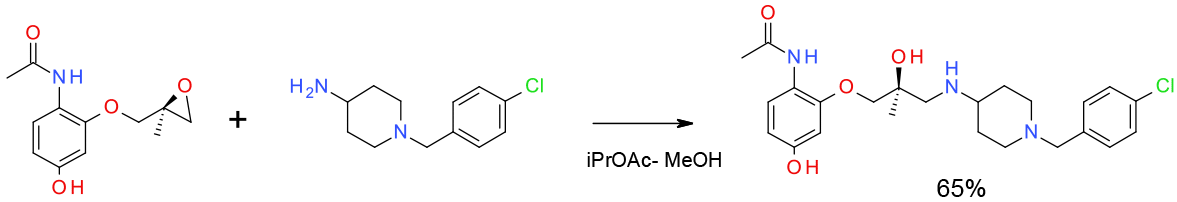
Experimental
Pilot plant scale
Org. Process Res. Dev. 2010, 14, 72–84
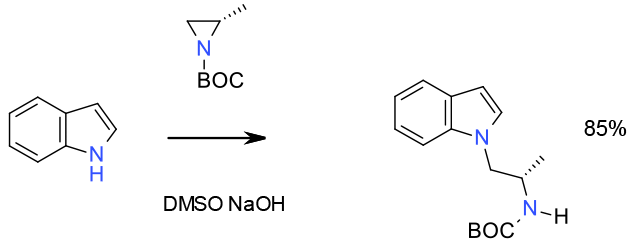
Experimental
gram scale
Org. Process Res. Dev. 2003, 7, 22-24
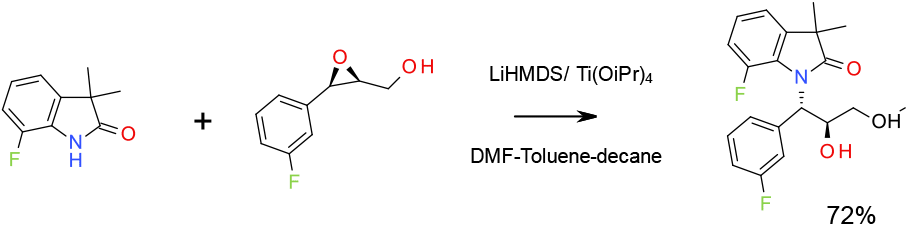
Experimental
2.5 Kg scale
Org. Process Res. Dev. 2009, 13, 880–887
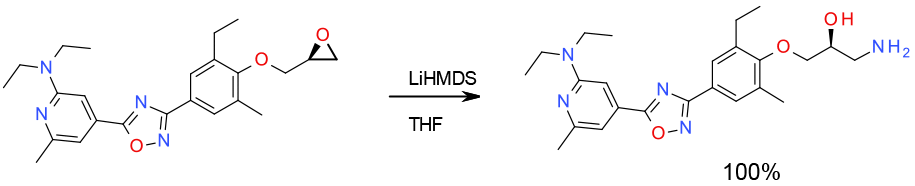
Experimental
50 gram scale
Org. Process Res. Dev. 2012, 16, 595−604
Green Review
-
Atom efficiency (by-products Mwt)
Epoxides are highly atom efficient electrophiles since there is no leaving group generated as a waste product - Safety Concerns
Epoxides are highly reactive. Low Mwt. epoxides should always be treated as thermally hazardous until proved otherwise – low Mwt. highly functionalised epoxides can decompose violently, occasionally when concentrated. The epoxide functionality will generate a positive PGI alert, and apart from possible long term effects, low Mwt. epoxides like ethylene oxide, propylene oxide and epichlorohydrin are also acutely highly toxic and need to be handled accordingly. - Toxicity and environmental/aquatic impact
The epoxide group is very reactive, and the resulting diol function is unlikely to give rise to ecotoxicity/ persistence. Any concerns here will be related to the bulk properties of the molecule containing the epoxide group. - Cost, availability & sustainable feedstocks
Many smaller epoxides are readily available at low cost, some now have the potential to be manufactured from renewable feedstocks. Many alkenes are available and can be epoxidized using green technology. - Sustainable implications
A highly atom efficient reaction, with some epoxides available from renewables.