Mitsunobu
Mechanism + Description
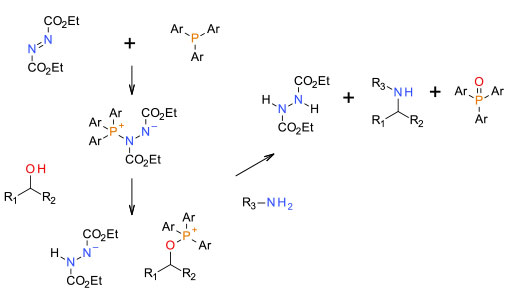
‘In situ’ activation of alcohol followed by SN2 displacement by amine
General comments
A reaction often used to construct amines via in situ activation of alcohols. Often quoted as not being scalable, many scaled examples have been reported. Generally very clean and high yielding, very efficient SN2 inversion of stereochemistry are attractive features. Detractions are very poor atom economy, the need to remove the by-products, and the azodicarboxylate diesters used as activators can be potent sensitizers and have process safety concerns. The Mitsunobu reaction is often used with ammonia equivalents to prepare primary amines. The high reactivity of the activated alcohol means that non-nucleophilic nitrogen centres like sulfonamides or ureas can be used as N sources. Various attempts have been made to ‘green’ the Mitsunobu reaction, but most still suffer from very poor atom economy or use of undesirable solvents, reagents etc.
Key references
Org. Process Res. Dev. 1999, 3, 460-464 Synthesis of a Spiro[cyclohex-1,1¢-isobenzofuranyl] A Dopamine Receptor Antagonist
Org. Process Res. Dev. 2010, 14, 868–877 Pilot-Plant-Scale Synthesis of an Alkylated Dihydrobenzothiadiazole S,S-Dioxide: Incorporation of a Late-Stage Mitsunobu Reaction
J. Am. Chem. Soc. 2006, 128 (30), 9636–9637 Organocatalytic Mitsunobu Reactions
Org. Process Res. Dev. 2008, 12, 831–836 A Potent R-Mannosidase II Inhibitor
Chem Review 2009, 109, 2551-2651Mitsunobu and Related Reactions: Advances and Applications
Angew. Chem. Int. Ed. 2013, 52, 4613–4617 Catalytic Mitsunobu reactions
Relevant scale up example
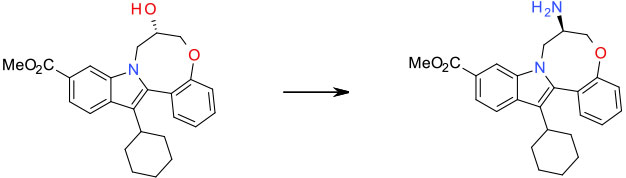
Experimental – Ph3P(O)N3 / DIAD/Ph3P/THF 9 kg scale
Org. Process Res. Dev. 2011, 15, 1116–1123
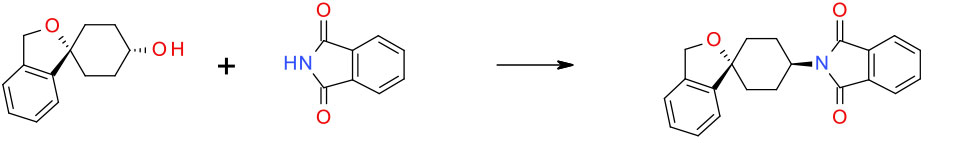
Experimental THF/Ph3P/DIAD/Phthalimide 20 g scale
Org. Process Res. Dev. 1999, 3, 460-464
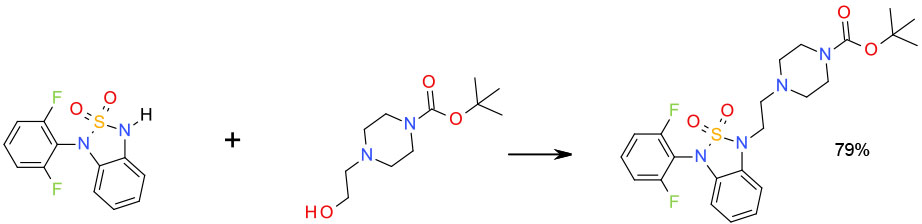
Experimental DIAD/Ph3P/THF 6 Kg scale
Org. Process Res. Dev. 2010, 14, 868–877
Experimental THF/Ph3P/ DIAD/TMSN3
Org. Process Res. Dev. 2008, 12, 831–836
Green Review
-
Atom efficiency (by-products Mwt)
Atom efficiency is very poor generating Ph3PO (278) and the corresponding hydrazone of DIAD / DEAD (or related activator). - Safety Concerns
The Mitsunobu reaction can be scaled. Major safety concerns are around the azodicarboxylate reagents which are toxic and potentially explosive. If making primary amines, and using azide and azide donors – NaN3 or Ph3P(O)N3 – main hazard is the thermal stability of azides and the potential to generate HN3 gas and the resulting explosion hazard. - Toxicity and environmental/aquatic impact
High Mwt hydrophobic phosphines, present a moderate hazard to the aquatic environment and may bio-accumulate. - Cost, availability & sustainable feedstocks
DEAD/DIAD May have limited availability and transport issues for large scale use – need to be shipped as solutions in toluene. - Sustainable implications
A transformation that has very poor atom economy. There may be also a considerable solvent PMI burden in removing by-products after the reaction.