Phase Transfer Catalysis
Mechanism + Description
Phase transfer catalysis (PTC) is a very useful technique to employ in the synthesis of amines via SN2 alkylation
PTC often allows the use of weaker, more environmentally friendly inorganic bases compared to organic and organometallic bases. More importantly, PTC can allow the use of a wider range of solvents other than dipolar aprotics. The application of PTC can allow very concentrated or neat reactions if reagents are liquids. A technique of very wide utility, substrate scope extends to aliphatic nucleophiles and heterocycles. PTC is scalable and readily carried out at industrial scale
Greenness
- Phase Transfer Catalysis offers the greenest variant of the SN2 reaction
- Use of more benign solvents (toluene, MTBE) instead of dipolar aprotic solvents
- Higher productivity- many examples have been carried out under solvent-free conditions
- Use of inorganic bases (NaOH, carbonate) instead of bulky organic bases (diisopropylethylamine, DBU, lithium hexamethyldisilazide, etc.)
General comments
The "q-value" and "C#" are two empirical parameters which are used to characterize the structure of quaternary ammonium salts for structure-activity relationships. C# is simply the total number of carbons on the 4 alkyl chains. Thus, the C# for methyl tributyl ammonium is 1 + 4 + 4 + 4 = 13. As the C# increases, the organophilicity increases and usually results in a higher concentration of the quat-anion pair in the organic phase. When the rate determining step is the organic phase reaction ("I-Reaction"), C#’s in the range of 16 to 32 often provide desirable reactivity. The "q-value" parameter is often useful when the rate determining step is mass transfer ("T-reaction"), especially when using hydrophilic anions such as hydroxide (about half of PTC applications fall in this category). The q-value is calculated by adding the reciprocals of the number of carbons on each of the four chains of the quat. Thus, the q-value for methyl tributyl ammonium is 1/1 + 1/4 + 1/4 + 1/4 = 1.75. For T-reactions, quats with q-values in the range of 1 to 2 often promote desirable reactivity, with q-values of 1.5 to 1.75 often being the best.
http://www.phasetransfer.com/04tipJul.htm
Quaternary ammonium phase-transfer catalysts sometimes experience "poisoning" by highly polarizable or lipophilic leaving groups, most notably iodide and tosylate. In one application, an alcohol was converted to a sulfonate ester for displacement. Using the mesylate gave 95% yield whereas using the tosylate gave 5% yield! These are both good leaving groups, however the quat pairs more strongly with tosylate than mesylate which in turn hinders the ability of the quat to transfer and react the desired nucleophilic anion. Guideline: Consider bromide instead of iodide and mesylate instead of iodide.
http://www.phasetransfer.com/04tipNov.htm
Key references
Books
E. V. Dehmlow, S. S. Dehmlow, “Phase Transfer Catalysis,” 3rd edition VCH Publishers, NY (1993)
C. M. Starks, C. L. Liotta, M. Halpern, “Phase Transfer Catalysis,” Chapman and Hall, NY, (1994). C-N bond formation, pp. 400-410; Industrial considerations, pp. 626-638.
M. Halpern, “Process Chemistry in the Pharmaceutical Industry,” K. G. Gadamasetti, Ed (1999), CRC Press, p. 283-296
Y. Goldberg, “Phase Transfer Catalysis” Gordon and Breach Science Publishers, 1992. pp. 25-91 and 101-126 for C-N bond discussion. Excellent summary of C-N bond forming reactions prior to 1992
General Phase Transfer Catalysis C-N bond formation references
Org. Process Res. Dev. 2008, 12 , 698–709 – PTC in OPRD: An Illustrative Overview
Angew. Chem. Int. Ed. 2013, 52, 4312–4348 Recent Developments in Asymmtric Phase Transfer Reactions
Angew. Chem. Int. Ed. 2007, 46, 4222–4266 Recent Advances in Asymmetric Phase-Transfer Catalysis
Topics in Catalysis 2004, 29, 145-161 Insight into Green Phase Transfer Catalysis
AIChE 1998, 44, 612-646 Phase Transfer Catalysis: Chemistry and Engineering
Relevant scale up example
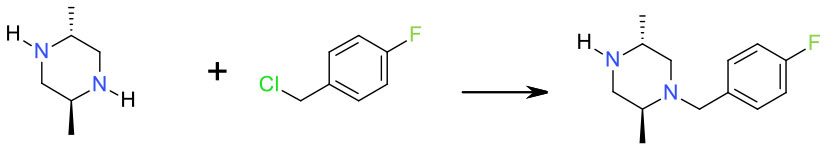
Experimental
Cyclohexane/ H2O/ NaOH/ nBu4NCl 25 Kg scale
Org. Process Res. Dev. 2007, 11, 754-761
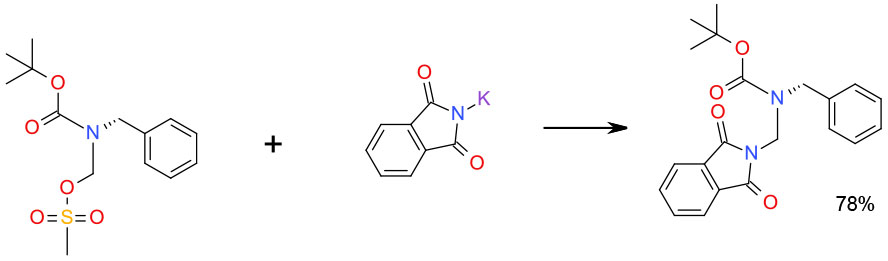
Experimental
Isopropyl acetate/ nBu4NBr
Org. Process Res. Dev. 2010, 14, 1254–1263
Green Review
-
Atom efficiency (by-products Mwt)
Depends on catalytic efficacy and recovery or disposal of the catalyst - Safety Concerns
Quats with hydroxide counter ions are corrosive and cause burns. Generally few safety concerns above normal processing hazards. - Toxicity and environmental/aquatic impact
Generally lower Mwt. PTC ‘s are preferred since these have less ecotoxicity in the aqueous environment. - Cost, availability & sustainable feedstocks
Many PTC’s are available in bulk at low cost. Most are derived from petrochemicals. - Sustainable implications
Biggest benefits are in the replacement of dipolar aprotic solvents with safer, more sustainable solvents with simple work-ups (avoiding large volumes of aqueous waste) and in improving efficiency.