Hydroiodic Acid (HI)
Mechanism + Description
Reduction occurs when treated with hot HI. Mechanism is probably electron transfer – protonation
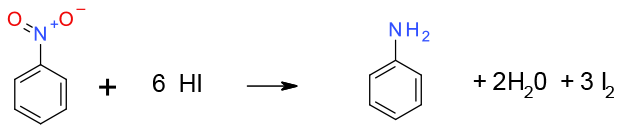
General comments
Reaction is generally selective in the presence of other reducible functional groups (not compatible with hydrolysable groups) but requires a lot of HI and thus is poor on atom economy. Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. High LCA reagent, although it is possible to recover iodide from waste materials.
Key references
Synthesis 2006, 18, 3127 Reduction of nitroarenes with HI
Phosphorus, Sulfur, and Silicon and the Related Elements 2004, 179, 1711
Relevant scale up example
None found.