Precious Metal Catalyst (PMC) or Nickel Catalyzed Hydrogenation
Mechanism + Description
The nitro group is reduced by coordination next to absorbed H2 on the active face of a metal particle on a solid support. The mechanism is complex and goes via a number of intermediates – see scheme
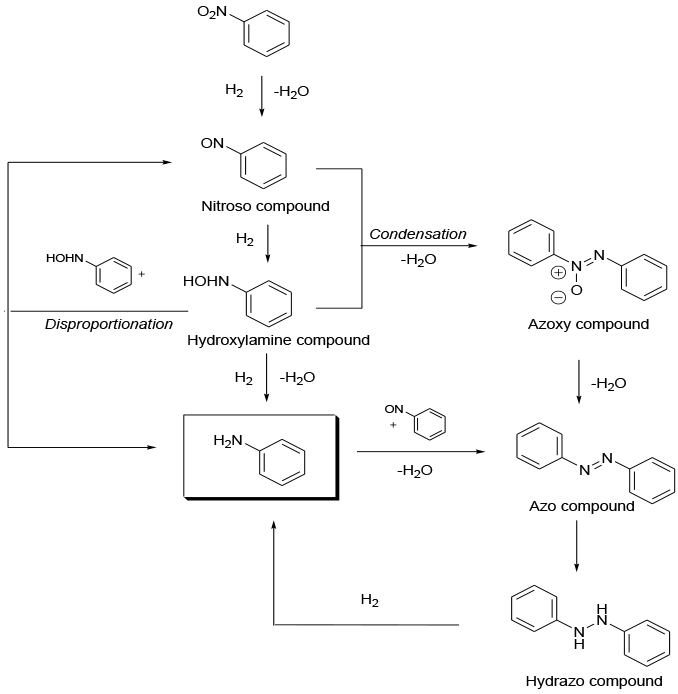
General comments
The most important intermediate in the catalytic hydrogenation of nitro-groups is the hydroxyamine species. Nitro compounds with electron-withdrawing substituents, e.g., sulfonamide or halogen can accumulate arylhydroxylamines in large amounts during the reaction. This is especially critical when the hydrogenation is carried out in batch reactors at low or medium temperatures.
Hydroxylamines are problematic in many respects. They are thermally unstable and can disproportionate with a strong exotherm, causing explosions. They are also known as potent carcinogens and therefore are hazardous in case of interrupted or incomplete hydrogenation. Hydroxylamine accumulation can cause poor product quality through condensation with the nitroso compound leading to formation of colored azo or azoxy products.
The accumulation of hydroxylamines can be diminished by the use of Vanadium promoters. These promote the reaction of hydroxylamine with the nitroso intermediate to give 2 moles of amine plus water.
Ni is most commonly utilized as Raney Nickel – a very common catalyst for nitro reduction. It is cheaper than PMC and can be advantageous where chemo selectivity is an issue. Nickel catalysts can also give better performance when hydroxylamine accumulation is an issue.
The pyrophoric nature of most Raney –type catalysts is the major concern, although several non-pyrophoric formulations can be sourced. Raney Nickel can be used in closed loop reactors, with automatic recycling.
Key to success in many transformations is getting selectivity in multi-functionalized molecules. This can be achieved by screening for the most appropriate catalyst and support – and by the use of additives like MgO, P(OPh)3 or morpholine – see examples.
Key references
Angew. Chem. Int. Ed. 1990, 29, 1169 and Chem. Commun. 1998, 119 Reductions of aliphatic nitro compounds to aliphatic amines
Org. Process Res. Dev. 2004, 8, 469 Selective catalytic hydrogenations of nitro compounds containing multiple functionalities
Advanced Synthesis & Catalysis, 2008, 350(3), 406 Novel palladium-on-carbon/diphenyl sulfide complex for chemoselective hydrogenation
J.Med.Chem. 2010, 53, 6768 hydrogenation using Pd(OH)2 catalyst
Angew. Chem. Int. Ed., 2008, 47, 888 and J. Org. Chem. 2000, 65, 8114 use of Pt/ C catalysts
Angew. Chem. Int. Ed., 1981, 20, 397 Ni catalyzed reduction of aliphatic nitro alcohols to amino alcohols
Organic & Biomolecular Chemistry, 2013, 11(17), 2827 chemoselective reduction of nitro groups with Raney Nickel
J. Org. Chem. 2010, 75, 3495 Reduction of NO2 in presence of iodide using V doped Pt/C
Tett. Lett. 2010, 51 (39), 5181 Reduction of halonitroaromatics using silver and gold catalzsed hydrogenations
Relevant scale up example
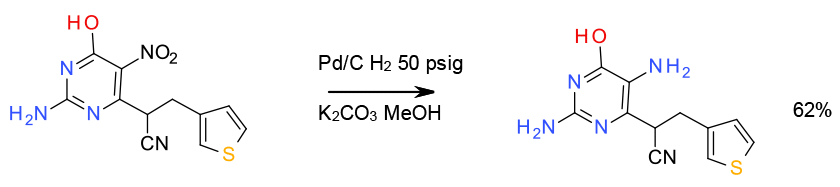
Reduction with Pd/C and H2 – 400 Litre scale
Org. Process Res. Dev. 2001, 5, 216
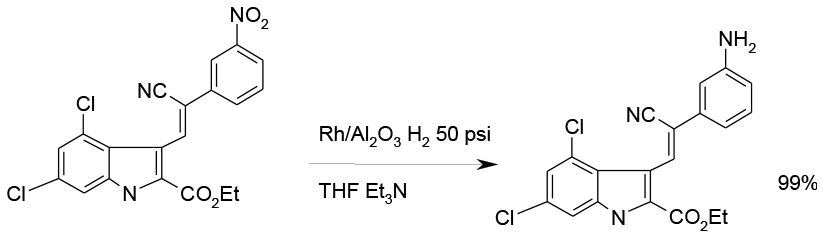
Selective hydrogenation with Rh on Al2O3 Kg scale
Org. Process Res. Dev. 2000, 4, 477
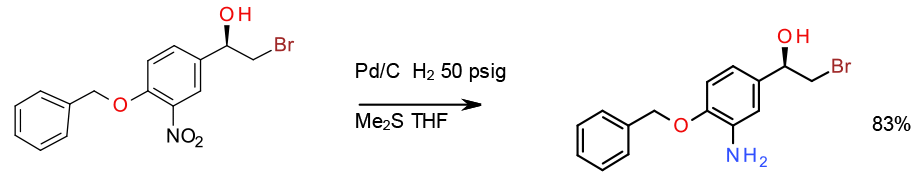
Selective hydrogenation with Pd/C poisoned with trace Me2S Kg scale
Org. Process Res. Dev. 2000, 4, 567
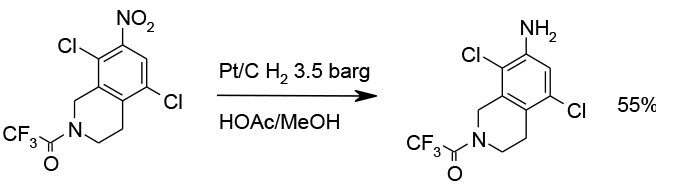
Chemoselective reduction with Pt/C, 13 Kg scale
Org. Process Res. Dev. 2010, 14, 108
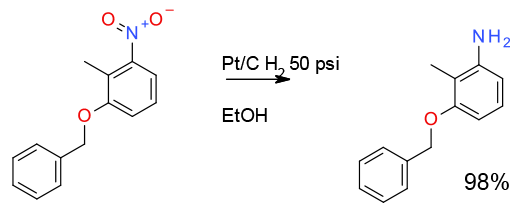
2.5 litre scale
Org. Process Res. Dev. 2007, 11, 30
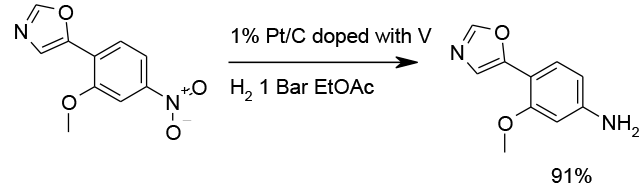
V doped Pt catalyst to avoid hydroxylamine accumulation
Pilot plant scale
Org. Process Res. Dev. 2010, 14, 1512
Ni reduction
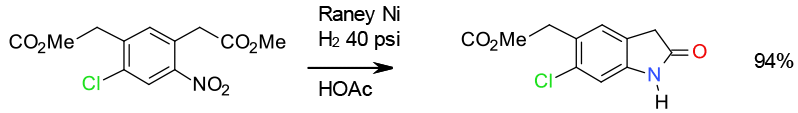
Ni hydrogenation – minimises hydroxylamine
and more robust to impurities 7 Kg scale 30 gallon
Org. Process Res. Dev. 2003, 7, 309
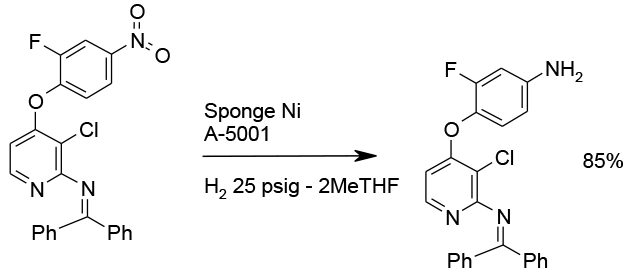
Chemoselective reduction with Raney Nickel.
Minimises hydroxylamine levels 2000 litre scale
Org. Process Res. Dev. 2012, 16, 1665
Green Review
-
Atom efficiency (by-products Mwt)
With optimized catalytic efficiency, PMC hydrogenation is an atom efficient process generating 2 moles of water as a by-product. - Safety Concerns
Typified by normal hazards around hydrogenation reactions. Catalytic transfer hydrogenation may avoid the need to handle H2 gas, but these reactions may generate H2 in situ. Reduction of nitrobenzenes is a highly exothermic reaction. Dry PMC and Ni hydrogenation catalysts can be pyrophoric and are usually handled water-wet – mixtures of solvent and catalyst in the presence of air can ignite. Appropriate care needs to be taken after processing in handling spent catalyst, and this can be recycled.
Some Pt salts used as catalyst precursors are strong sensitizers.
ICH guidelines for metals in human medicinal products. - Toxicity and environmental/aquatic impact
Main concern is around solubilisation and loss of precious metal/ heavy metal catalysts into waste streams. Most PMC/Base metal levels are tightly regulated. The same applies to potential carry through into the API- see next slide. Some Ni salts are sensitizers and carcinogens and listed on the EU SVAH list – http://echa.europa.eu/candidate-list-table. This is of less concern for metallic hydrogenation catalysts. - Cost, availability & sustainable feedstocks
H2 is cheap and non-polluting and can be produced from renewable resources. - Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with efficient recovery and recycle. Pd is most commonly used precious metal for hydrogenolysis and this is rated at high risk of depletion. Platinum group elements (Pt, Ru, Pd, Os, and Ir) are flagged as having a very high relative supply risk index value (2011 British geological Survey Risk list), No concern for abundant base metals like Ni.