Reduction with TiCl3
Mechanism + Description
Mild and efficient reagent for nitro reduction. Mechanism is electron transfer followed by protonation.
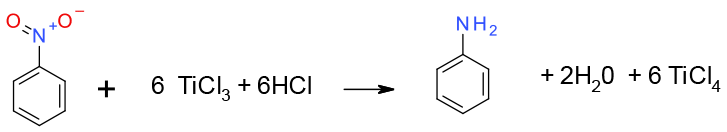
General comments
Low valent Ti complexes can be used to reduce nitro groups to amines. The most common reagent is a solution of TiCl3. Ti2+ reagents usually need to be prepared in-situ using amalgams which gives rise to environmental issues. TiCl3 is available commercially. On quenching, metal oxide residues can be problematic.
Key references
Synthetic Commun. 1983, 13, 495 Selective Reduction of Nitro Compounds With Titanium (II) Reagents
Molecules 2005, 10, 1318 reduction of the nitro group of 5-nitroimidazoles with TiCl3
Relevant scale up example
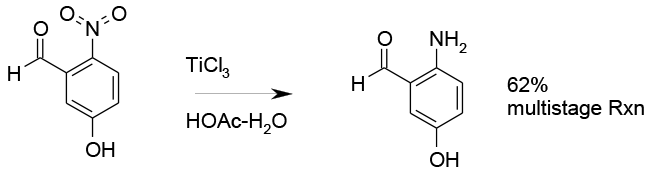
Selective reduction with TiCl3 2 litre scale
Org. Process Res. Dev. 1999, 3, 347