Sn2+ reduction
Mechanism + Description
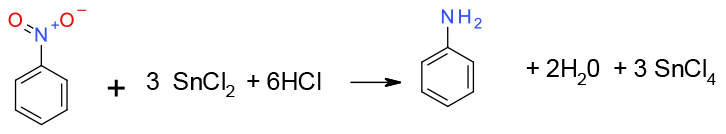
Similar to Fe or Zn metal – electron transfer from Sn2+ salt followed by protonation. Acids, water or alcohols can be used as the proton source
General comments
An older methodology for the synthesis of anilines. This method of reduction was often selected over catalytic hydrogenation due to compatibility issues with other functional groups in the molecule. However, today there are many selective catalysts that show excellent performance in the reduction of multi-functionalized nitroarenes.
Key references
J. Org. Chem., 1983, 48 (15), 2515 Effect of meta- and para-substituents on the stannous chloride reduction of nitrobenzenes in aqueous ethanol
Tett. Lett. 1984, 25, 839 Selective reduction of aromatic nitro compounds with stannous chloride
J. Med. Chem. 2000, 43, 3344 reduction of nitro aromatics with SnCl2
Relevant scale up example
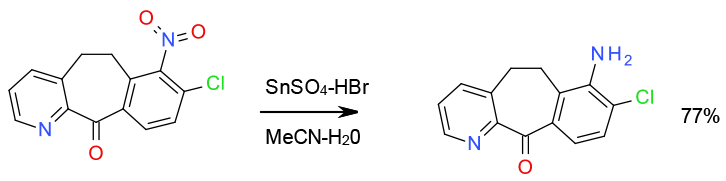
Selective reduction with in situ generated SnBr2 77 Kg scale SnBr2 generated in situ to reduce cost
Org. Process Res. Dev. 2003, 7, 692
Green Review
-
Atom efficiency (by-products Mwt)
Moderate to poor using 3 moles of Sn2+ salt and giving 3 moles of a Sn4+ salt as by-product. - Safety Concerns
No great concerns above the exothermic reaction. SnCl2 is a suspected sensitizer. Quenching reactions leads to hydrate Sn oxides which can be difficult to remove from the product/ reactor. - Toxicity and environmental/aquatic impact
Organotin compounds are very toxic to both humans and the environment. There is much less concern with inorganic Tin salts, although like most metals, levels in water systems have control limits. Levels need to be monitored in the API. - Cost, availability & sustainable feedstocks
Historically a cheap reagent depending on the anion and grade – cost rising with increasing scarcity of Sn - Sustainable implications
Sn is becoming at moderate to high risk of depletion