Stoichiometric Reduction with Low Valent Sulfur (sulfide/dithionite)
Mechanism + Description
A number of inorganic sulfur reagents can be used to reduce aliphatic and aromatic nitro compounds to amines such as sodium sulfide, sodium hydrogen sulfide and sodium dithionite. Mechanism is via electron transfer – shown for dithionite.
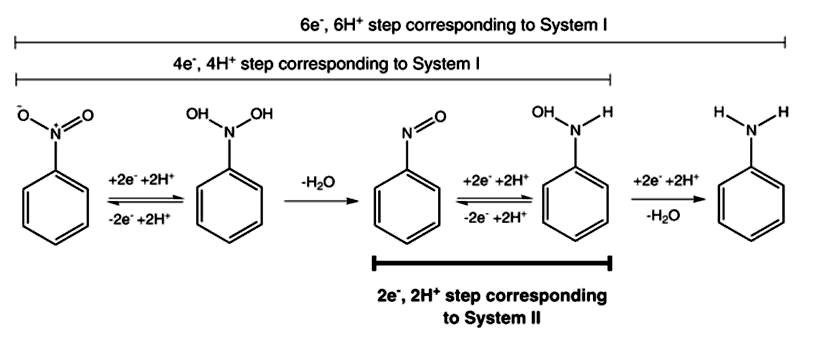
General comments
Reagents are heated with nitro compound. Ethanol-water is a common solvent system.
Key references
Tetrahedron Lett. 1988, 29, 635 Sodium sulfide has been used for the selective reduction of aromatic nitro group in the presence of aliphatic nitro groups
J. Med. Chem. 2005, 48, 5104 and J. Med. Chem. 2007, 50, 4453 reduction of nitro aromatics with sodium dithionite
Chemistry Letters 2008, 37 (5), 544 and J. Med. Chem. 2009, 52, 1275 reduction of nitroarenes with hydrogen sulfide salts
Relevant scale up example
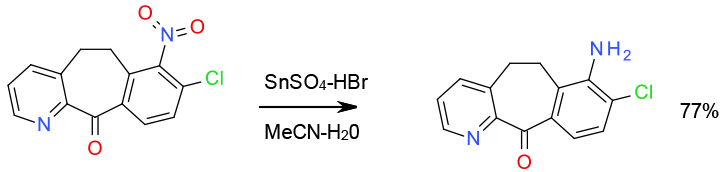
Imidazole formation following reduction with Na2S2O4 Gram scale Na2S2O4 process safety information given
Org. Process Res. Dev. 2012, 16, 96
Green Review
-
Atom efficiency (by-products Mwt)
Overall moderate – Dependent on sulfur salt used. High Mwt. of many S salts is due to polyhydrate formation. - Safety Concerns
Use of sulfur compounds can give rise to issues with odour and toxic gases (H2S) if treated with acids. Dithionite is unstable and it’s decomposition temperature can be considerably lowered in contact with organic solvents. - Toxicity and environmental/aquatic impact
These sulfur reagents are of concern in fresh water ecosystems. Even low levels can give rise to odor issues. - Cost, availability & sustainable feedstocks
Generally readily available and cheap. - Sustainable implications
None.