Stoichiometric Zn or Fe Metal Reduction
Mechanism + Description
Treatment of nitro compound with a reducing Metal Fe, Zn, Sn in the presence of acid (HCl/HOAc, NH4Cl) gives the aniline plus oxidized metal salts. The Mechanism proceeds via electron transfer to give radical anions followed by protonation
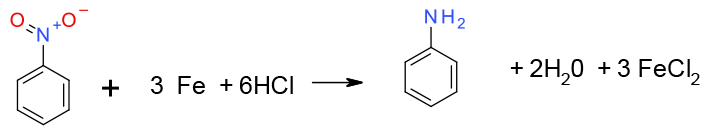
General comments
Metals like Zn, Fe & Sn were common reagents used before the advent of PMC and Ni hydrogenation.
These are often used in excess and in the presence of acids. Sometimes other metal salts are added as promoters. All dissolving metal reductions must be treated with particular care, as particle size and stirring rate can be crucial to the rate of reaction and potential exotherm.
This method of reduction was often selected over catalytic hydrogenation due to compatibility issues with other functional groups in the molecule. However, today there are many selective catalysts that show excellent performance in the reduction of multi-functionalized nitroarenes.
Key references
Synlett 2010 (20): 3019-3022 reduction of nitrobenzenes with Fe powder and CaCl2
J. Org. Chem., 2004, 69 (4), 1262–1269 reduction of aliphatic Nitro compounds to amines with Zn/HCl/IPA
J. Med. Chem., 2011, 54, 3524–3548 Reduction of nitro groups with Zn/HOAc
J. Med. Chem., 2010, 53, 3502–3516 reduction of nitro groups with Zn/NH4Cl
Relevant scale up example
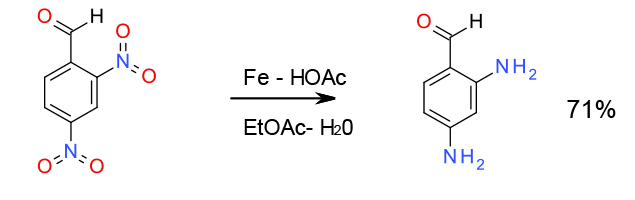
Selective reduction with Fe – 100’s gram scale
Org. Process Res. Dev., 2001, 5 (5), 539
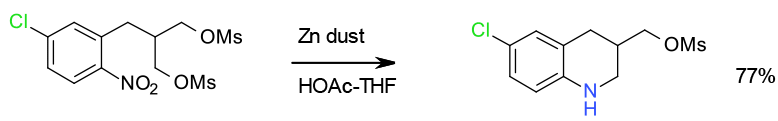
Selective Zn reduction – 300 Kg scale
Org. Process Res. Dev. 2010, 14, 1110
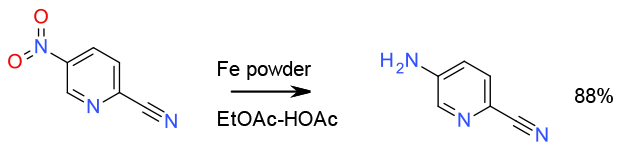
Reduction with Fe 100 gallon scale
Org. Process Res. Dev. 2004, 8, 62
Green Review
-
Atom efficiency (by-products Mwt)
Moderate producing two molecules of water and a metal salt as by-products, however, acids and metals are usually used in excess. - Safety Concerns
Finely divided metals can be pyrophoric. These reactions are usually run by adding metal to the reaction mixture and delayed exotherms can present a hazard. Considerable down stream processing can be involved in removing metal and metal salts from the product and reactor. Recovered unreacted metals from these reductions can present a hazard in that they can continue to evolve hydrogen for some time after being removed from the reaction. - Toxicity and environmental/aquatic impact
Iron, Zinc and Tin have quality levels in waste water effluent and levels need to be monitored in the API. - Cost, availability & sustainable feedstocks
Very cheap and readily available reagents. - Sustainable implications
Fe is abundant but Zn and Sn are at moderate to high risk of depletion.