List of Reagents
Mechanism + Description
Similar to metal-catalzsed reduction with hydrogen, except H2 is generated in situ from a hydrogen donor. Catalysts can be heterogeneous or homogeneous –homogeneous metal catalysts can be reduced in situ to metallic catalysts, sometimes nano-particulate in nature. Donors generally fall into two categories – those that decompose continuously on the catalyst surface to generate hydrogen gas like formate, or those that act as nascent sources of hydrogen in the presence of a reducible group – cyclohexene
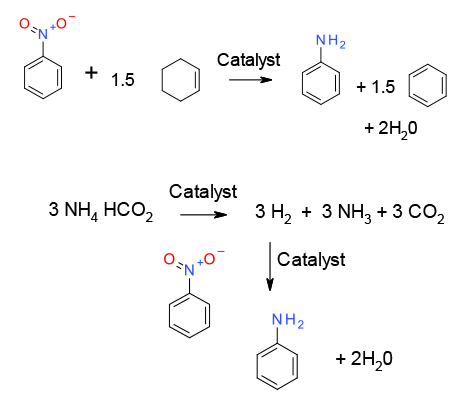
General comments
A wide range of hydrogen donors and metal catalysts can be used for hydrogenation if a dedicated hydrogenation facility is not available. As with H2 gas, selectivity is possible with multi-functional molecules with correct choice of catalyst and donor molecule – see examples section. The most common catalyst for transfer hydrogenation is Pd, but cheaper base metals like Fe and Ni can also be used as catalysts. Caution needs to be exercised with some metals such as Zn, Mg. these are often labelled as ‘catalytic’, but most examples use 2-3 molar equiv. of metal.
Key references
Chemical Reviews, 1985, 85(2), 129 Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds- A review with 310 refs on transfer hydrogenation
Advanced Synthesis & Catalysis, 2005, 347(14), 1769. New trends in palladium-catalyzed transfer hydrogenations using formic acid
J. Org. Chem. 2007, 72, 6599Pd−Carbon -Induced Catalytic Transfer Hydrogenation with Triethylsilane
Org. Lett., 2005, 7,(22)5087 Use of silanes and siloxanes with KF/Pd(OAc)2 to reduce aliphatic and aromatic nitro compounds
Tetrahedron Lett. 1988, 29, 5733 reduction of nitro compounds using ammonium formate as a hydrogen source
J. Org. Chem. 1977, 42, 3491 Palladium catalyzed reductions of halo- and nitroaromatic compounds with triethylammonium formate
Synth. Lett. 2006, 7, 1043 Transfer reduction with methylcyclohexene
J. Am. Chem. Soc. 2011, 133, 12875 General and Selective Iron-Catalyzed Transfer Hydrogenation of Nitroarenes without Base
Relevant scale up example
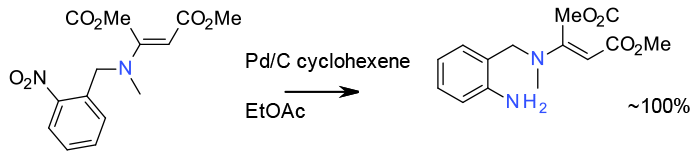
Transfer hydrogenation – Pd/C cyclohexene. 2000 Litre scale
Org. Process Res. Dev. 2003, 7, 655
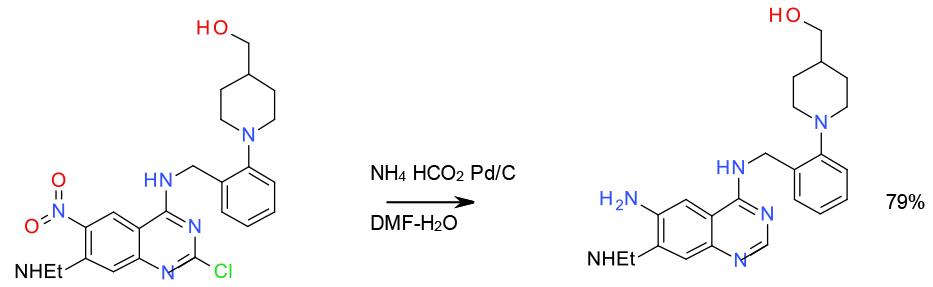
Transfer hydrogenation – nitro reduction and deschlorination 100 gram scale
Org. Process Res. Dev. 2001, 5, 426
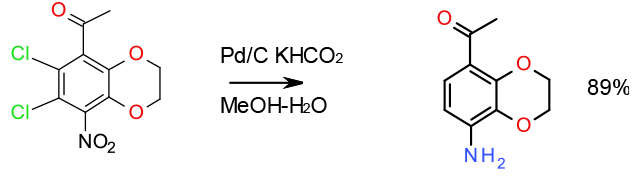
Transfer hydrogenation – nitro reduction and deschlorination in the presence of ketone KHCO2 used instead of NH4 HCO2 to avoid issues with sublimation 100 gallon scale
Org. Process Res. Dev. 2001, 5, 116
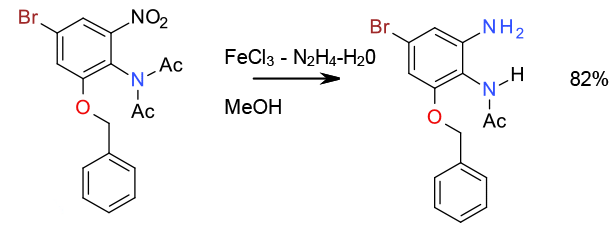
Transfer hydrogenation – Selective reduction and deprotection (deacylation) with FeCl3/Hydrazine 11 Kg/150 Litre scale
Org. Process Res. Dev. 2008, 12, 1170
Green Review
-
Atom efficiency (by-products Mwt)
Can vary from good to poor depending on the Mwt of the donor, and the excess above stoichiometric that has to be employed. The by-products are water and the oxidized donor and related by-products – isopropanol/acetone, hydrazine/N2, cyclohexene/ benzene, formate/ CO2 etc. Occasionally, large excesses of donors are used due to unproductive production and loss of H2 or to increase reaction rate. - Safety Concerns
Reactions can produce H2 gas and thus produce a flammable atmosphere above the reaction. Safety attributes of the individual donors/ by-products need consideration –
Hydrazine, Boric acid, benzene – (suspect) carcinogens
Borohydride will generate hydrogen gas on quenching - Toxicity and environmental/aquatic impact
Generally good – ammonia and phosphate may cause issues with discharge into water. Higher Mwt terpenes and siloxanes may bioaccummulate. - Cost, availability & sustainable feedstocks
Most H2 donor molecules are readily available and cheap - Sustainable implications
Some H2 donors can be obtained from sustainable/ biorenewable sources – isopropanol, formate, terpenes