Pyridine Ring Synthesis
The inclusion of an article in this document does not give any indication of safety or operability. Anyone wishing to use any reaction or reagent must consult and follow their internal chemical safety and hazard procedures and local laws regarding handling chemicals
Users need to ensure that reagents/chemicals are available at the right scale/cost for individual requirements. For legal reasons, recommendations for suppliers cannot be made in these guides. Many free to use and fee for service search engines are available for chemical sourcing.
General Overview
This guide focuses on the construction of the pyridine ring system rather than manipulation of pyridines.
The pyridine ring is a very common structural heterocyclic motif appearing in pharmaceuticals, agrochemicals. Simple pyridines like picolines are made in the bulk chemical industry used catalyzed reactions of aldehydes / ketones and ammonia at high temperature and pressure, often in flow/gas phase reactions. Carbon-carbon bonds are formed by aldol type reactions, reaction with ammonia, cyclisation and oxidation/ elimination to produce the aromatic heterocycle. Simple pyridines can also be extracted from natural products like coal tar.
A wide range of different catalytic and stoichiometric reactions have been utilized for the synthesis of pyridine rings. Some of the most common disconnections are shown in the scheme below:
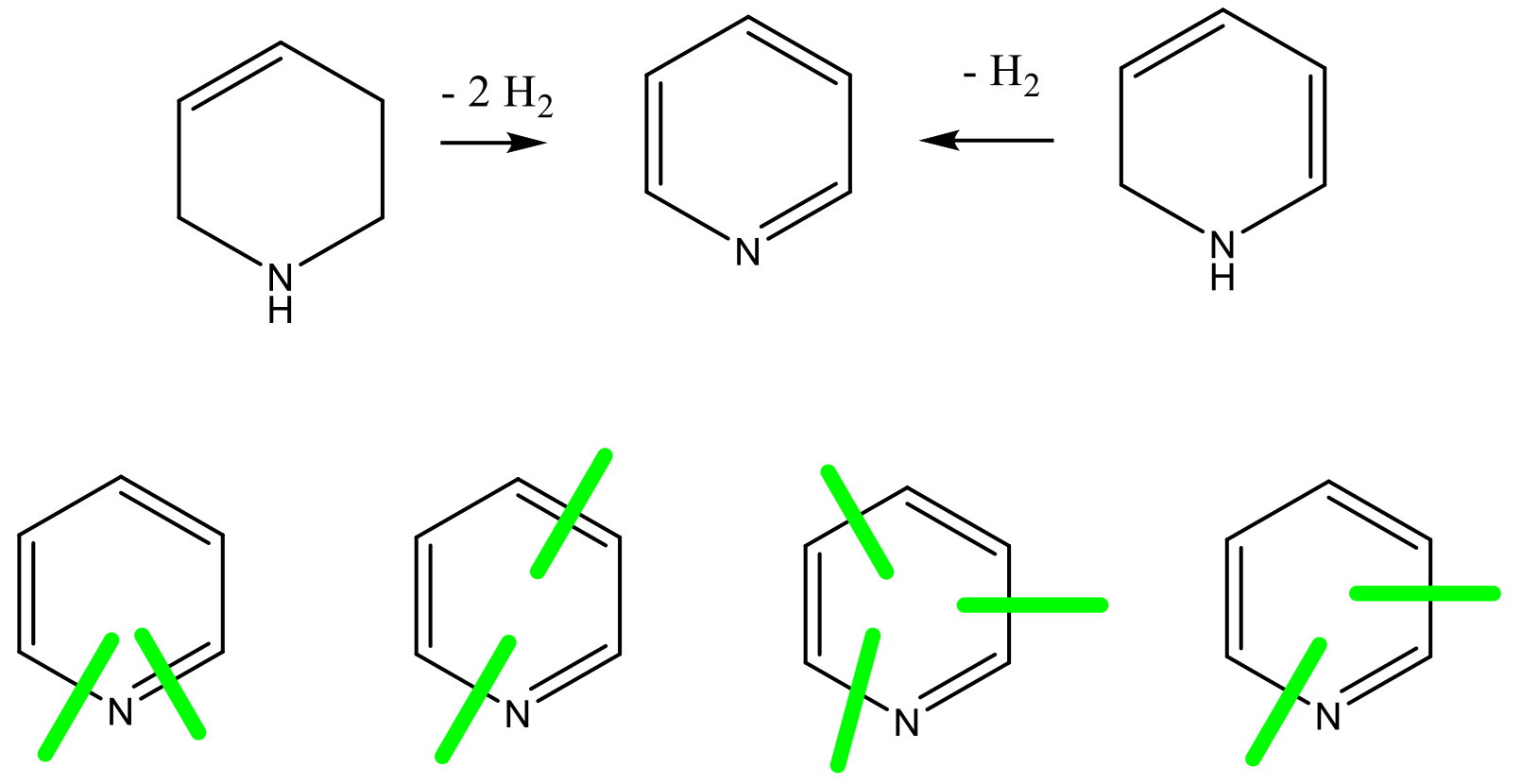
A review of ~600 papers in Organic Process Research & Development (OPR&D) revealed very few described syntheses of the actual pyridine ring. Thus, for pyridines, the case would appear to be very similar to fluorinated synthons—for scaled syntheses, starting materials with the basic features in place are brought in from fine/bulk chemical manufacturers and manipulated to more advanced intermediates for use in the final product. The source of the pyridine ring is not disclosed, and the vast bulk of chemistry reported is functionalization for substituent manipulation or reaction(s) at the pyridine ring carbon atoms.
General Literature Reviews: Achiral Hydrogenation
Scriven, E. F. V. (ed) Pyridines: From Lab to Production. Elsevier, 2013.
Levy, S. L.; Othmer, D. F. Synthesis of pyridines. Ind. Eng. Chem. 1955, 47, 789–796.
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th Edition. Wiley-Blackwell, 2013.
Other web-based resources:
https://www.organic-chemistry.org/synthesis/heterocycles/pyridines.shtm
Green Criteria for Pyridine Ring Synthesis
- If possible, uncatalyzed multi-component reactions are preferred.
- Direct formation of the aromatic system by elimination of a small molecule, e.g., water, alcohol, etc., is preferential to having to oxidize a dihydropyridine.
- Oxidation of tetra/dihydropyridines should use atom efficient green oxidants—O2/H2O2 preferred over heavy metals and poor atom efficient oxidants like Oxone™ and DDQ.
- One pot or telescoped processes are preferred over long linear sequences with multiple isolations.
- Large molar excesses of reagents should be avoided if possible.
- Solvents with CMR properties/alerts should be avoided.
- If using catalytic methods, base metals (e.g., Cu, Ni, Fe) should be considered in preference to Ir, Ru, Pd or other precious metal catalysts.