Condensation/ Multi-component Reactions MCR/Oxidation Approaches to Pyridines
Mechanism + Description
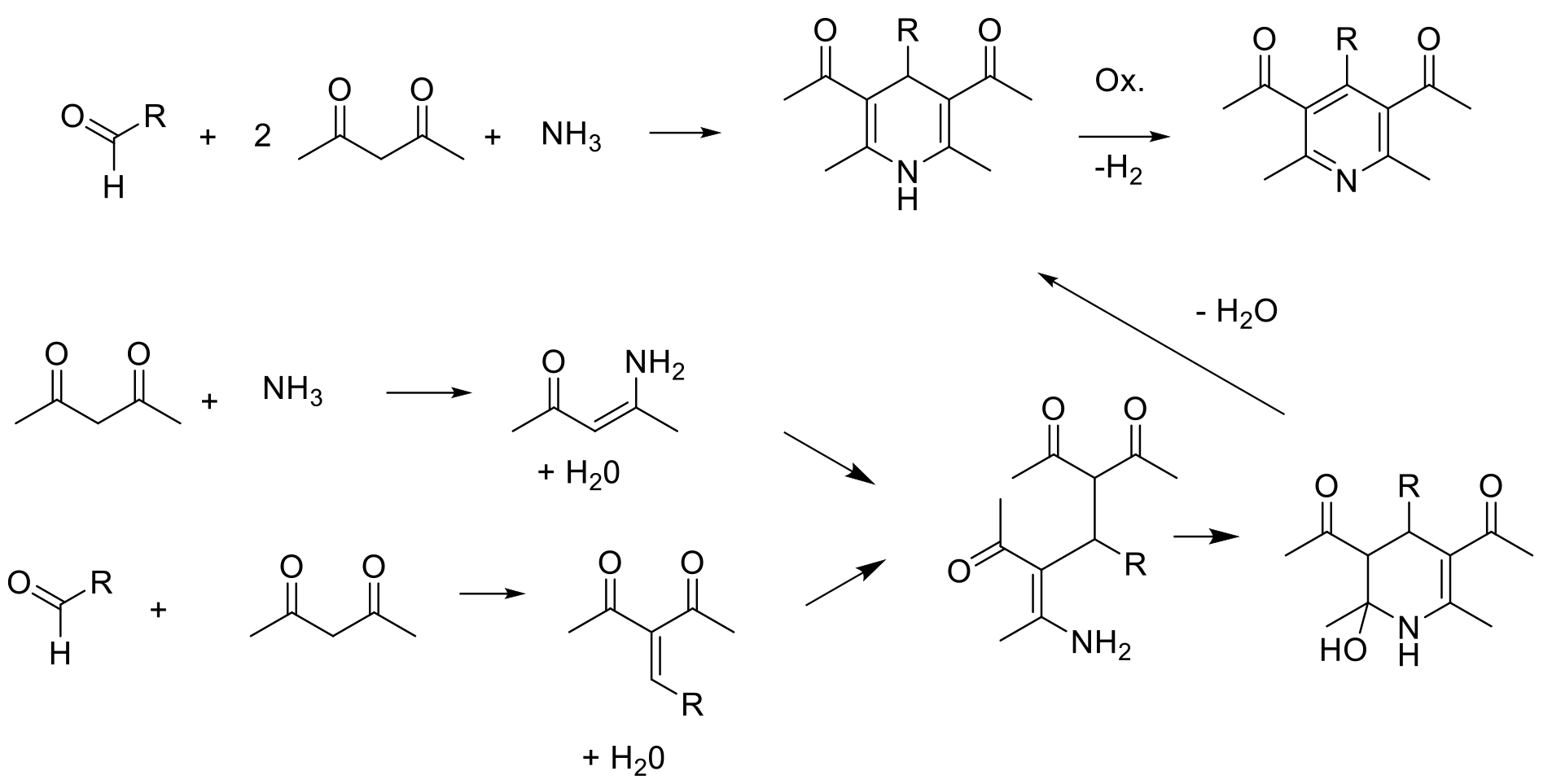
Hantzsch-type pyridine synthesis
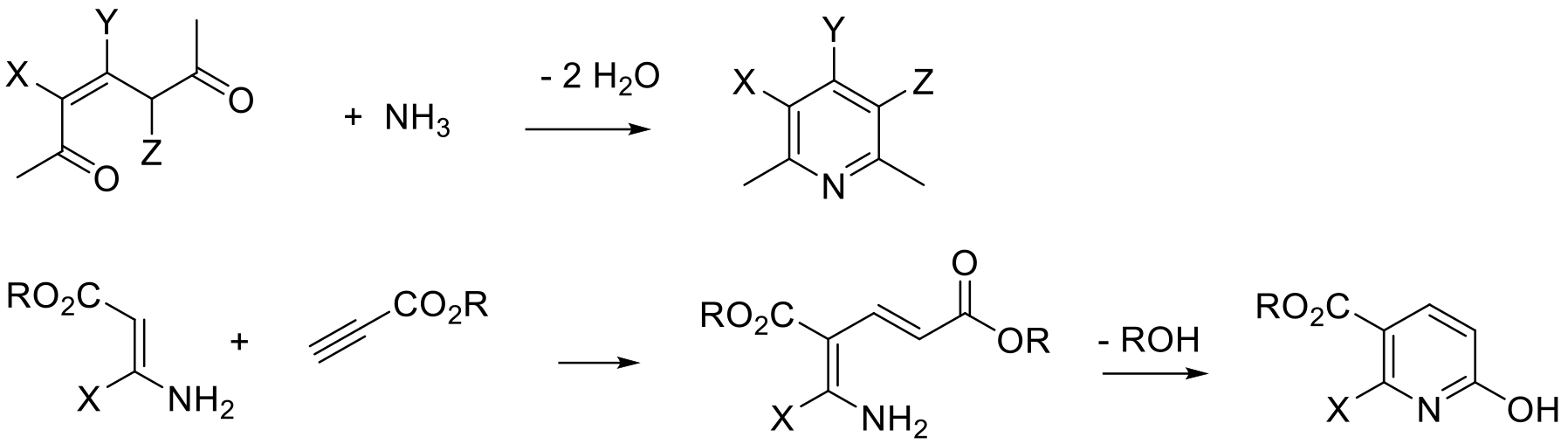
Bolhmann-Rahtz pyridine synthesis
Construction of carbon skeleton then ring closure by addition / elimination
General comments
The construction of pyridines using condensation reactions/addition-elimination reactions is widely used to make a wide range of functionalized pyridines. These reactions can be run as linear sequences to build up the precursor to ring formation, or more commonly run as multi-component reactions (MCR) in which the precursor to cyclisation is built up from simple reactive synthons and amines. Typically, carbon-carbon bonds are formed by Michael or aldol type reactions. This can be done via sequential additions of reagents or as ‘one pot’ type processes—all in.
Exemplar pyridine ring forming reactions are:
- Guareschi−Thorpe reaction
- Bohlmann−Rahtz reaction
- Hantzsch reaction
- Kröhnke reaction
The two main routes are used: Hantzsch-type, which gives rise to a tetrahydropyridine product that requires an oxidation reaction to give the product pyridine, and Guareschi−Thorpe / Bohlmann−Rahtz approaches that give the aromatic pyridine directly by substitution/elimination of small molecules like water, alcohols or amines.
Key references
Phillips, A. P. Hantzsch’s Pyridine Synthesis. J. Am. Chem. Soc. 1949, 71, 12, 4003–4007.
OXIDATION
Zard, S. Z. The xanthate route to pyridines. Tetrahedron 2020, 76, 130802.
Scale-up examples – condensation/ Multi-component reactions MCR/Oxidation approaches to pyridines
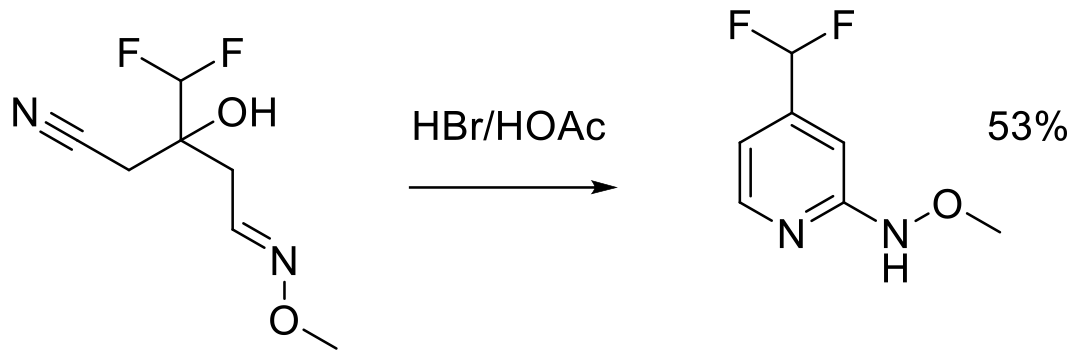
Org. Process Res. Dev. 1997, 1, 370-378.
2 kg scale
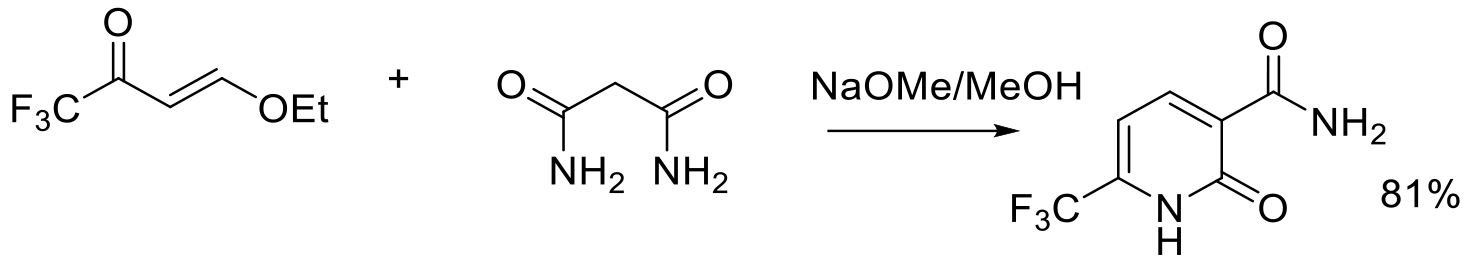
Org. Process Res. Dev. 1997, 1, 370-378.
2 g scale
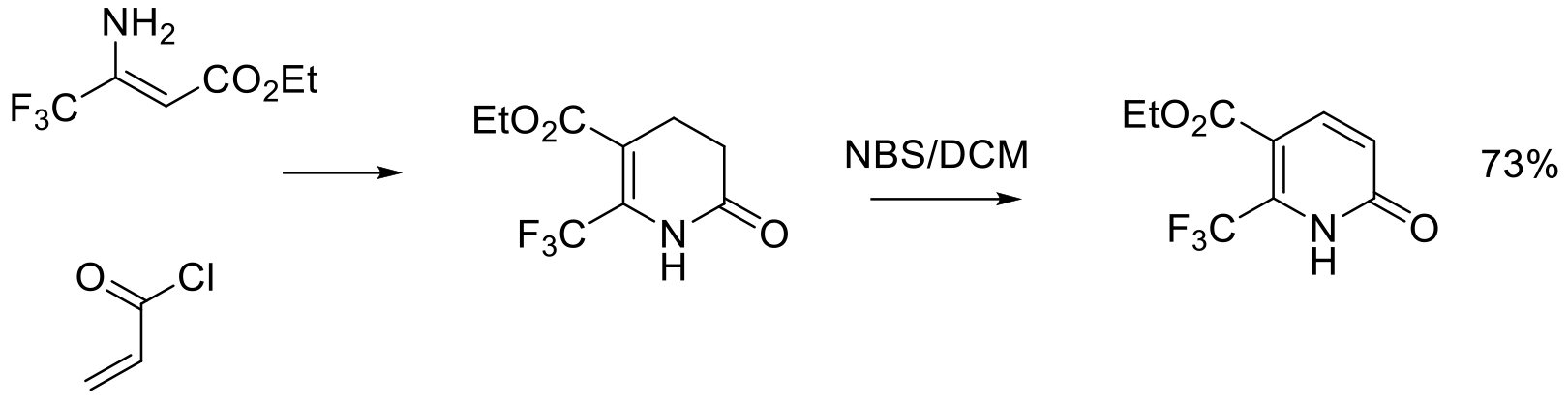
Org. Process Res. Dev. 2015, 19, 454−462.
800 g scale
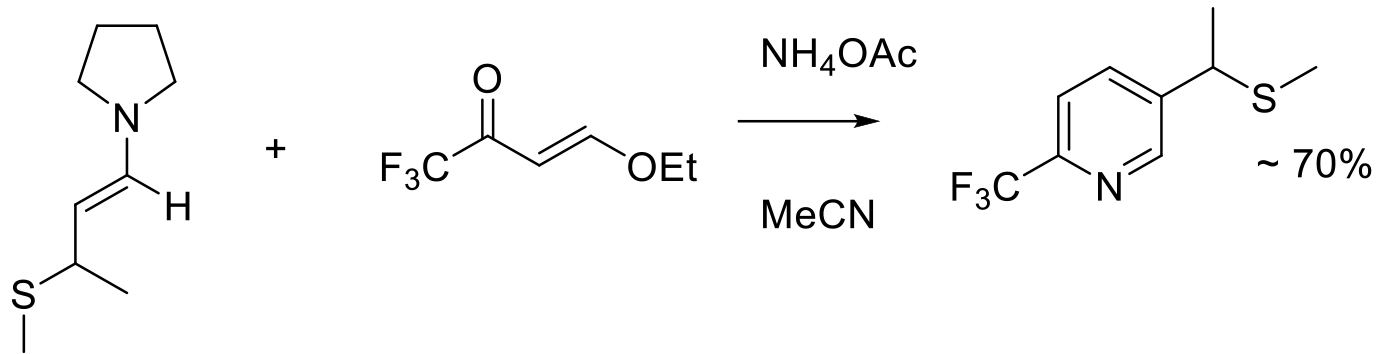
Org. Process Res. Dev. 2020, 24, 1763–1771.
2 kg scale
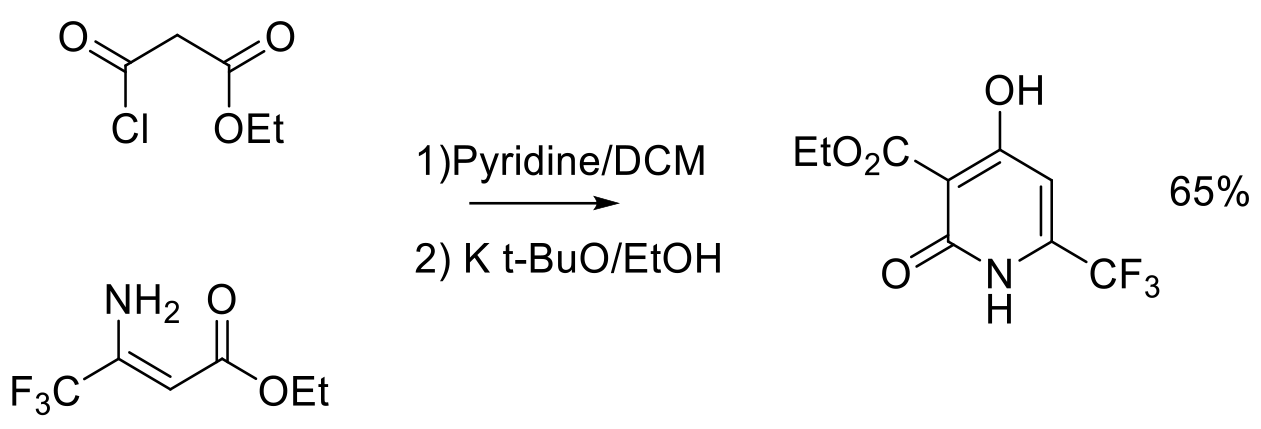
Org. Process Res. Dev. 2011, 15, 788–796.
150 g scale
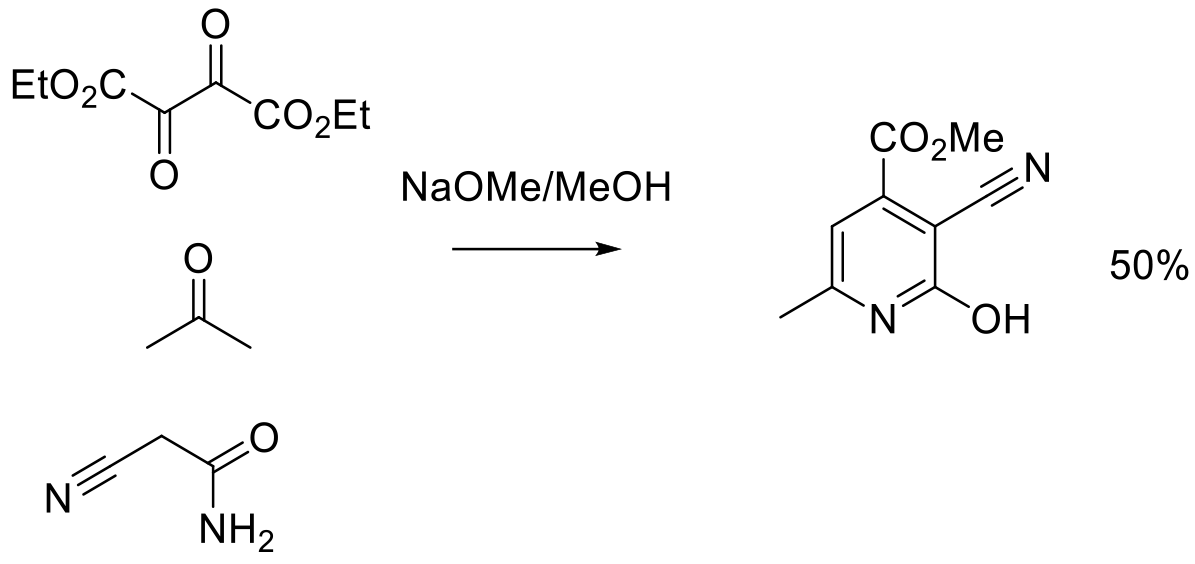
Org. Process Res. Dev. 2012, 16, 595−604.
400 g scale
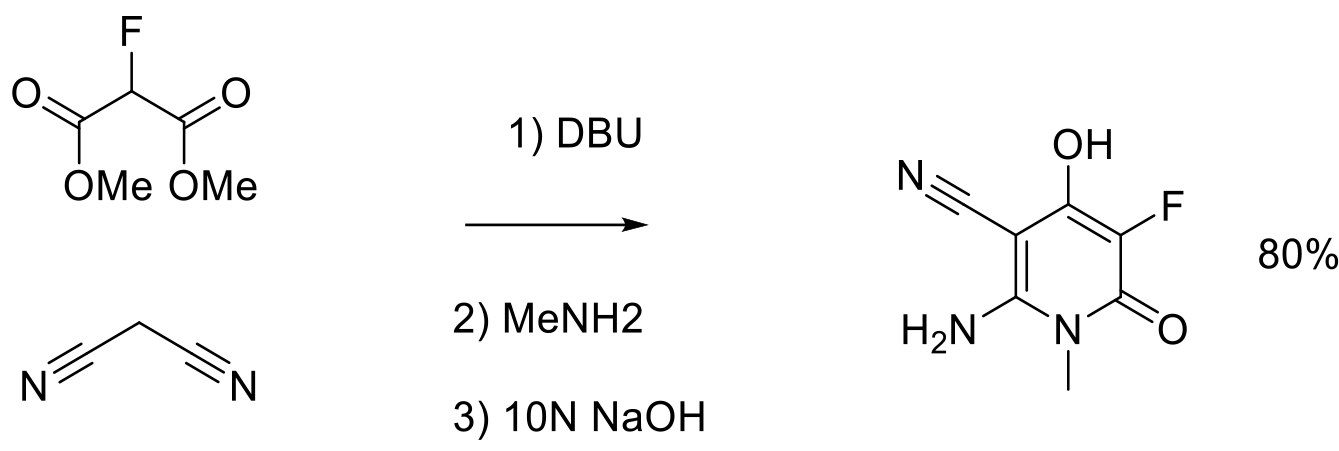
Org. Process Res. Dev. 2012, 16, 1652−1659.
3 kg scale
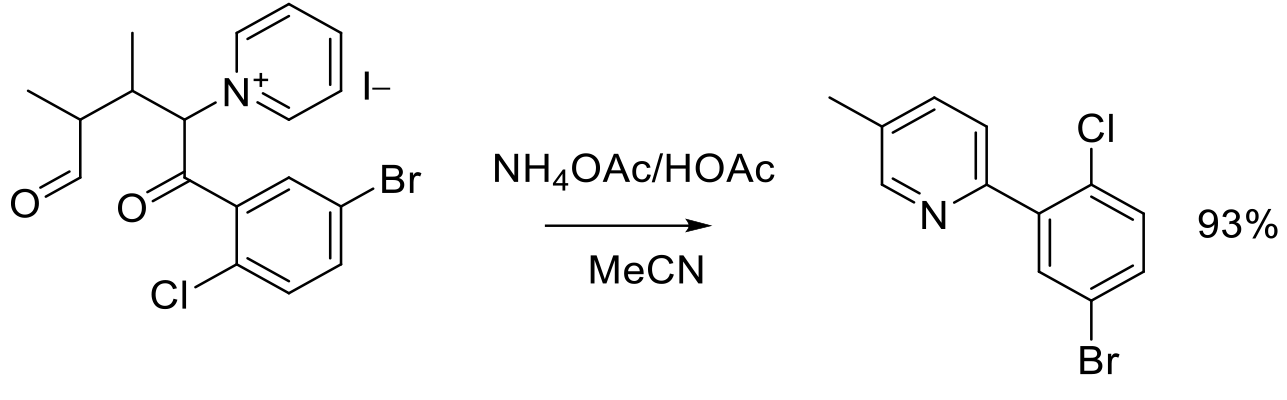
Org. Process Res. Dev. 2012, 16, 1739−1745.
300 g scale
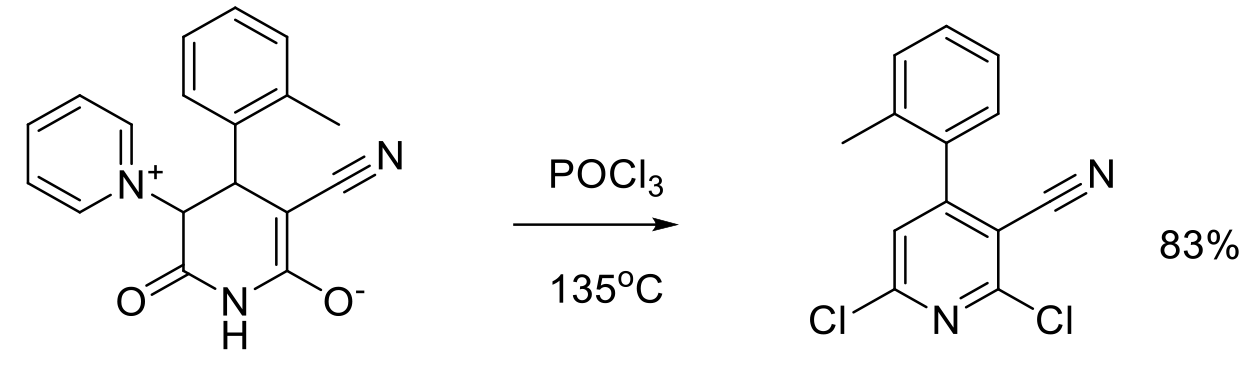
Org. Process Res. Dev. 2006, 10, 1157–1166.
60 g scale
Green Review – Condensation/ Multi-component reactions MCR/Oxidation approaches to pyridines
- Atom efficiency (by-products, molecular weight)
Generally good efficiency and atom economy with simple by-products like water, ethanol, methanol, etc.
In general, MCR reactions to give pyridine products can be slow with moderate yields, but these syntheses can be amenable to techniques like microwave heating or flow chemistry to improve conversion time and yields. - Safety Concerns
No major concerns.
Alkynes and alkenes are high-energy compounds and appropriate safety studies should be carried out before scale-up, especially with one pot or all in reactions.
Hydroxylamine and hydrazine are potentially explosive and highly toxic in the case of hydrazine.
When oxidizing a Hantzsch intermediate, key concerns are safe operation with flammable organic solvents (air/hydrogen peroxide and other reagents that can generate O2), and the potential of oxidizing agents to form unstable mixtures with some reagents/solvents. Oxygen in the presence of air can form an explosive mixture, and measures should be taken to limit the O2 concentration below the limit that would support combustion. - Toxicity and environmental/aquatic impact
Main concern is around loss of precious metal/ heavy metal catalysts into waste streams. Most precious and heavy metal levels are tightly regulated. The same applies to potential metal carry-through into the API. Hydrophobic, high mol. weight phosphines and phosphine oxides can be persistent and bioaccumulative and should not be discharged into aqueous waste streams.
Relevant regulatory guidance on permitted metal levels in API’s should be consulted.
Metals Removal Guide
Guidance.pptx
There may be issues with discharging aqueous waste with high NH3 content. Ammonia is harmful to the aquatic ecosystem. - Cost, availability, & sustainable feedstocks
Most reagents are cheap and readily available on scale. Most are probably still produced from petrochemical feedstocks. - Sustainable implications
No major concerns. Some pyridines can be produced from bio renewable feedstocks, but not in great structural diversity of scale as yet.