Mechanism + Description
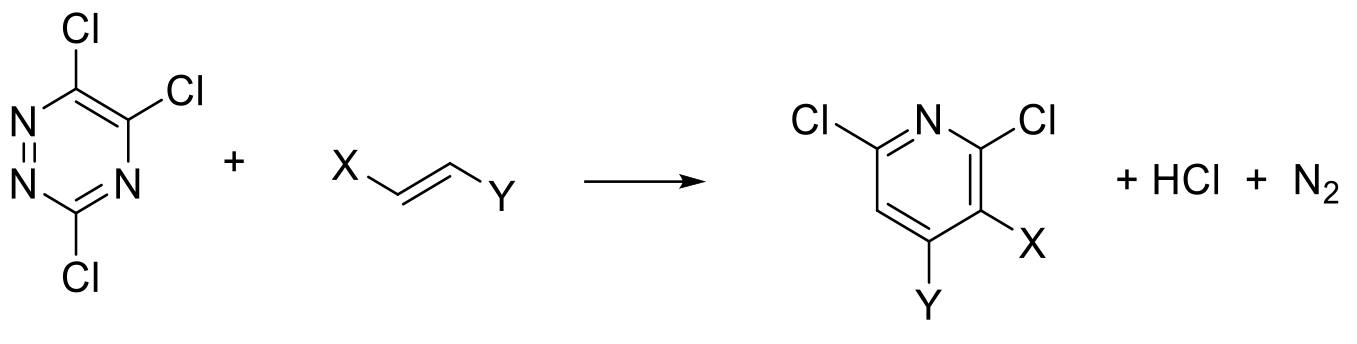
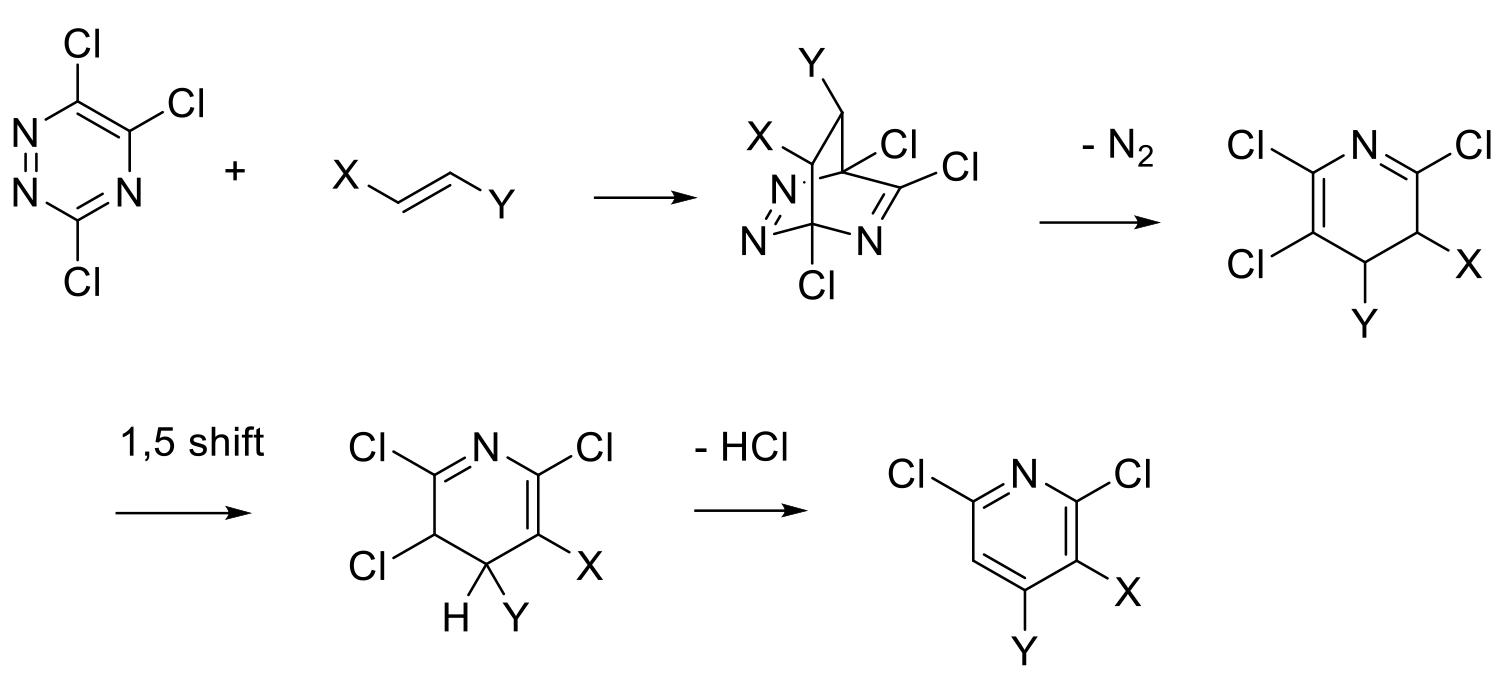
Diels-Alder type cyclisation to form a six-membered ring then an aromatization process to give the pyridine ring system.
General comments
A number of thermal cycloadditions are known to give tetrahydropyridines or pyridines as primary products. Normal electron-demand Diels-Alder reactions are known, but generally are difficult to achieve in high yield due to generally unfavorable electronics.
Both intermolecular and intramolecular versions have been reported to give pyridines. Intramolecular reactions generally being more facile and requiring less harsh conditions (i.e. lower temperature).
Most successful processes giving pyridines are of the inverse electron demand Diels-Alder type, with the eventual pyridine nitrogen as part of an electron poor diene system such as a 1,2,4 triazine or substituted pyrimidine, etc.
The initial Diels-Alder adduct generally extrudes a small neutral molecule like N2, RCN, CO2 to give a tetrahydropyridine which is further converted by rearrangement/elimination to the aromatic pyridine. If a facile aromatization process is not feasible, the intermediate tetrahydropyridines can react as dienes with any excess dienophile present.
Whilst the Diels-Alder/Aromatization step can be very atom efficient, not many diene and dienophile precursors are readily available as articles of commerce, and multi-stage sequences to prepare the starting materials can detract from the direct pyridine ring forming reaction.
Key references
Boger, D. L. Diels-Alder reactions of heterocyclic aza dienes. Scope and applications. Chem. Rev. 1986, 86, 781–793.
Coffinier, D.; El Kaim, L.; Grimaud, L.; Ruijter, E.; Orru, R. V. A. A new multicomponent reaction for the synthesis of pyridines via cycloaddition of azadienes and ketenimines. Tetrahedron Letts. 2011, 52, 3023–3025.
Wang, S.-W.; Guo, W.-S.; Wen, L.-R.; Li, M. A new approach to pyridines through the reactions of methyl ketones with 1,2,4-triazines. RSC Adv., 2014, 4, 59218–59220.
Nagata, T.; Obora, Y. Transition-Metal-Mediated/Catalyzed Synthesis of Pyridines, Pyrimidines, and Triazines by [2+2+2] Cycloaddition Reactions. Asian J. Org. Chem. 2020, 9, 1532–1547.
Boger, D. L.; Panek, J, S. Pyridine construction via thermal cycloaddition of 1,2,4-triazines with enamines: studies on the preparation of the biaryl CD rings of streptonigrin. J. Org. Chem. 1982, 47, 3763–3765.
Barlow, M. G.; Haszeldine, R. N.; Simpkin, D. J. Heterocyclic polyfluoro-compounds. Part 37. Diels–Alder reactions of trichloro- and trifluoro-1,2,4-triazine. J. Chem. Soc., Perkin Trans. 1, 1982, 1245-1249 .
Zhang, Y.; Luo, H.; Lu, Q.; An, Q.; Li, Y.; Li, S.; Tang, Z.; Li, B. Access to pyridines via cascade nucleophilic addition reaction of 1,2,3-triazines with activated ketones or acetonitriles. Chinese Chemical Letters, 2021, 32(1): 393-396.
Frissen, A. E.; Marcelis, A. T. M.; Geurtsen, G.; de Bie, D. A.; Van Der Plas, H. C. Intramolecular Diels-Alder reactions of 2-(alkynyl)pyrimidines and 2-(alkynyl)pyridines. Tetrahedron 1989, 45, 5151-5162.
Frissen, A. E.; Marcelis, A. T. M.; Van Der Plas, H. C. Ring-transformations of pyrimidines by intramolecular diels-alder reactions. Synthesis of annelated pyridines. Tetrahedron 1989, 45, 803-812.
Boger, D. L.; Ichikawa, S.; Jiang, H. Total Synthesis of the Rubrolone Aglycon. J. Am. Chem. Soc. 2000, 122, 12169–12173.
Villacampa, M.; Pérez, J. M.; Avendaño, C.; Menéndez, J. C. Ultrasound assisted Diels-Alder reactions of 1-azadienes with “normal” electronic demand. Tetrahedron 1994, 50, 10047-10054.

