Metal-catalysed Pyridine Ring Synthesis
Mechanism + Description
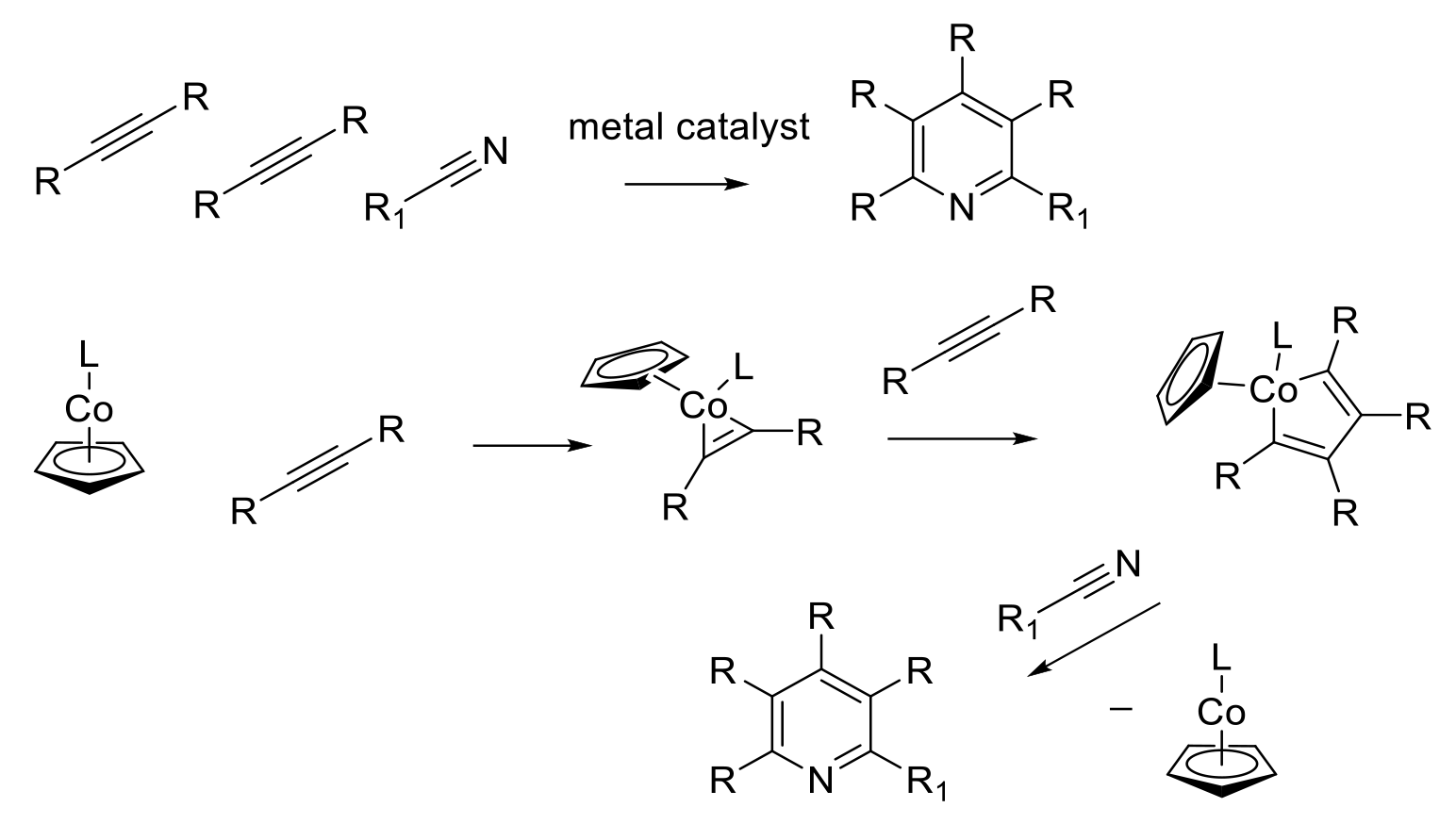
Use of metal catalysts to from pyridines from simple building block using pathways disfavored by normal thermal chemistry.
General comments
Metal catalysis is used in a wide range of pyridine chemistry:
- Oxidation of di and tetrahydropyridines to pyridines
- Function group interconversion on pyridine ring substituents
- Further annulation of existing pyridine rings into more complex heterocycles
A wide range of metal-catalyzed transformations are also known to form tetrahydropyridine and pyridine rings. Characteristically, these are very convergent and atom efficient. Examples being ring closing metathesis reactions and catalysis of cycloaddition reactions that do not occur or are too slow thermally. [2 + 2 + 2] cycloadditions are rarely used because they are enthalpy and entropy disfavoured barriers. Metal catalysis can provide alternative lower energy pathways, e.g. [2+2+2] cycloaddition of acetylenes and nitriles to form pyridine rings.
Key references
Donohoe, T. J.; Jones, C. R.; Barbosa, L. C. A. Total Synthesis of (±)-Streptonigrin: De Novo Construction of a Pentasubstituted Pyridine using Ring-Closing Metathesis. J. Am. Chem. Soc. 2011, 133, 16418–16421.
Donohoe, T. J.; Fishlock, L. P.; Basutto, J. A.; Bower, J. F.; Procopioub, P. A.; Thompson, A. L. Varela, J. A.; Castedo, L.; Saa, C. Scope of Ru(II)-Catalyzed Synthesis of Pyridines from Alkynes. J. Org. Chem. 2003, 68, 8595–8598.
Varela, J, A.; Saá, C. Construction of Pyridine Rings by Metal-Mediated [2 + 2 + 2] Cycloaddition. Chem. Rev. 2003, 103, 3787–3802.