Hydrogen Fluoride and Amine Complexes (HF-NR3)
Mechanism + Description
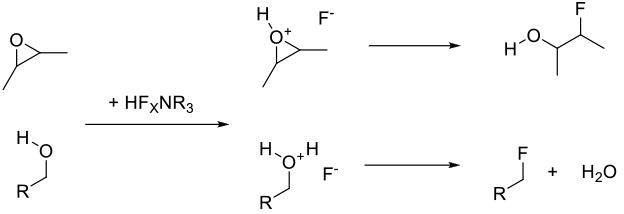
HF complexes work essentially as HF would – protonation of the substrate followed by nucleophilic addition of fluoride ion. Depending on the substrate, they may go formally via the carbocation.
General comments
Anhydrous HF is very volatile (b.p. 20°C), highly toxic, and extremely corrosive to all organs. It is used in bulk and as a fluorine source in electrochemical fluorination, but requires specialized plant and handling techniques. More commonly used are HF-amine complexes, which consist of an amine salt with additional HF. These tend to be liquids at ambient temperature and are much easier to handle. A number of these reagents have been used and some are commercially available. The most common is Olah’s salt Pyridine-HF(x) (PPHF) – Pyridinium Poly(hydrogen fluoride) (Pyridine ~30%, hydrogen fluoride ~70%). PPHF reacts with secondary and tertiary alcohols, alkenes, alkynes, epoxides, dithiolanes and chlorides. Substitution of F for NH2 is achieved by an in situ diazotization–fluorination sequence. Mixtures of Et3N and HF are often used as substitutes for Olah’s reagent and are available commercially.
Key references
Relevant scale up example
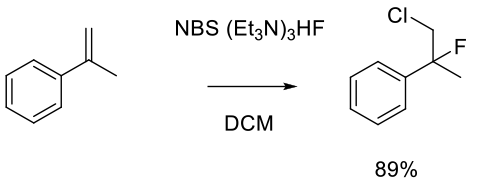
Haufe, G.; Alvernhe, G.; Laurent, A.; Ernet, T.; Goj. O.; Kröger, S.; Sattler, A. Org. Synth. 1999, 76, 159.
Experimental
7g scale
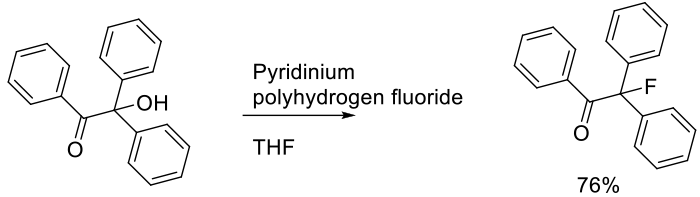
Kumar, A.; Pal, A. K.; Anand, R. D.; Singh, T. V.; Venugopalan, P. Tetrahedron 2011, 67, 8308-8313.
Experimental
<2g scale
Green Review
-
Atom efficiency (by-products Mwt)
A reasonable atom economy reagent – the addition of HF to double bonds, if 100% efficient, in the fluorination of alcohols, generates H2O (18) as a by-product. HF reagents are often used in large excess compared to the stoichiometric HF usage, so the use of any excess reagent should be minimized. - Safety Concerns
HF and related complexes can cause severe burns and corrode glass vessels/equipment.
Toxic Substances Portal – Hydrogen Fluoride (HF). Agency for Toxic Substances & Disease Registry. - Toxicity and environmental/aquatic impact
High concentrations of HF are extremely toxic to all life forms. Lower concentrations of fluoride can be harmful to aquatic life. Organofluorine compounds, especially polyfluorinated, can be persistent and bioaccumulate.
Camargo, J. A. Fluoride Toxicity to Aquatic Organisms: A Review. Chemosphere 2003, 50, 251-264. - Cost, availability & sustainable feedstocks
HF and HF-amine complexes are available in bulk - Sustainable implications
Fluoride sources are at medium risk of depletion and all fluorine is used in a dispersive manner. The high energy needed to produce F2 would make this a high LCI impact material.