PyFluor
Mechanism + Description
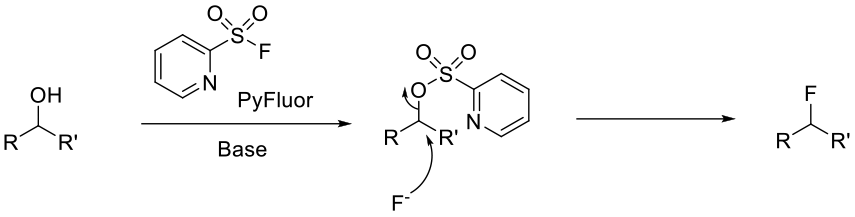
CPyFluor serves to activate the alcohol in the presence of base through the formation of the intermediate pyridinesulfonate, which can subsequently be displaced with fluoride.
General comments
PyFluor represents a low-cost deoxyfluorination reagent that exhibits high chemical and thermal stability. The reagent has been demonstrated to tolerate a wide range of functionality, and minimize competing elimination reactions, which often complicate purifications of deoxyfluorination processes. Reaction times are longer and require basic conditions, though the relative ease of preparation coupled with the enhanced safety profile of the reagent should enable the use of this methodology on scale.
Key references
Relevant scale up examples
None found.