Nuclophilic Fluorination by F–
Mechanism + Description
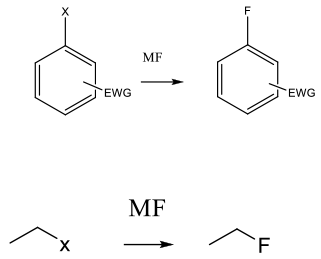
Traditional SN2 / SNAr mechanism displacing a leaving group. From an aryl/heteroaryl substrate, alkyl halide/activated alcohol or other electrophilic halide acceptor, e.g. epoxide.
General comments
Typical reagents for nucleophilic fluoride addition are simple metal salts. KF, CsF, KHF2 or tetraalkyl ammonium salts Like Me4NF, n-Bu4NF. TBAFanh fluorinates primary alkyl halides, tosylates, and mesylates within minutes at or below temperature. In polar aprotic solvents such as acetonitrile and DMSO, aromatic halides undergo halogen exchange (Halex) reactions with TBAFanh to form the corresponding fluoroaromatics and activated nitroaromatic compounds are fluoro-denitrated in high yield. Various functional groups, including aryl esters, aldehydes, ketones, and N-benzyl protecting groups, are compatible with these fluorination conditions. The nucleophilicity of commercial TBAF·xH2O in SNAr reactions is attenuated significantly compared to that of TBAFanh. Substrates are limited to strongly activated nitropyridines and nitroarenes. TBAF (tBuOH)4 complex has been shown to be a more reactive alternative. Phase transfer catalysis is often employed with metal fluoride salts. Epoxides can be opened enantioselectivily with fluoride anion and chiral Co catalysts. A classical route to aryl fluorides is via aryl diazonium salts and F- donors like BF4-/PF6– – the Balz–Schiemann reaction.
Key references
Ikunaka, M. PTC in OPRD: An Illustrative Overview. Org. Process Res. Dev. 2008, 12, 698–709.
Relevant scale up example
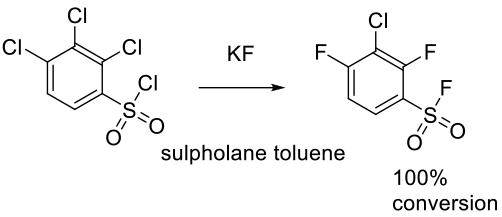
Moore, R. M. Org. Process Res. Dev. 2003, 7, 921-924.
Experimental
40g scale
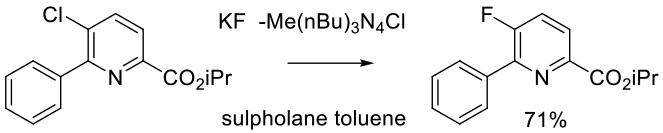
Allen, L. J.; Lee, S. H.; Cheng, Y.; Hanley, P. S.; Muhuhi, J. M.; Kane, E.; Powers, S. L.; Anderson, J. E.; Bell, B. M.; Rother, G. A.; Sanford, M.; Bland, D. C. Org. Process Res. Dev. 2014, 18, 1045−1054.
Experimental
150g scale
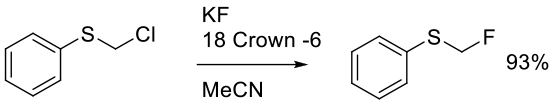
Prakash, G. K. S.; Shao, N.; Wang, F.; Ni, C. Org. Synth. 2013, 90, 130-144.
Experimental
10g scale
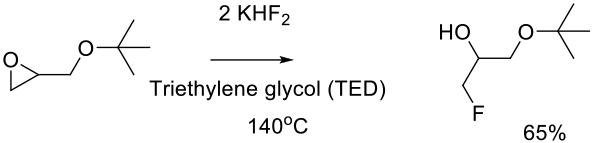
Adam, J.-M.; Foricher, J.; Hanlon, S.; Lohri, B.; Moine, G.; Schmid, R.; Stahr, H.; Weber, M.; Wirz, B.; Zutter, U. Org. Process Res. Dev. 2011, 15, 515–526.
Experimental
75kg scale
Green Review
-
Atom efficiency (by-products Mwt)
Reasonable atom economy, especially for simple alkali metal fluorides – less so for tetraalkylammonium reagents. Byproducts are simple salts where fluoride has been exchanged for the leaving group in the electrophile. - Safety Concerns
Materials compatibility with fluoride anion needs to be assessed. If acidified in work-up, HF can be generated. See section on HF and issues. - Toxicity and environmental/aquatic impact
High fluoride levels can be harmful to some aquatic organisms. Higher mol wt organic cations can be inhibitory or toxic to certain aquatic life forms, so caution needs to be exercised with aqueous wastes.
Camargo, J. A. Fluoride Toxicity to Aquatic Organisms: A Review. Chemosphere 2003, 50, 251-264. - Cost, availability & sustainable feedstocks
Most are available in bulk at reasonable cost. Simple potassium salts are preferred to Li or Cs and to organic cations. - Sustainable implications
These are poor atom efficiency reagents that come F2. They are high LCI materials.