Cl2 and Electrophilic Cl+ /Radical reagents
Mechanism + Description
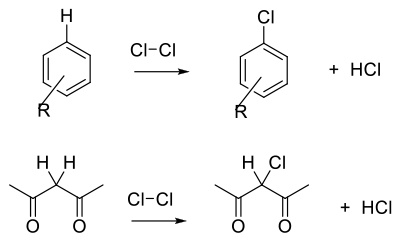
Cl2 and Cl+ equivalents are electrophilic reagents normally used to add to unsaturated C-C bonds, take part in electrophilic substitution reactions with aromatics/heteroaromatics, or react with acidic C-H bonds. Electrophilic and radical chlorine reagents are generally very reactive, and issues with selectivity may arise.
General comments
Due to the high reactivity of Cl2/Cl+ reagents, selectivity and over-chlorination can be problematic, and the choice of reagent needs consideration. To achieve optimum yield and selectivity, Cl2 is a strong electrophile and will react directly with electron rich double bonds, aromatics/hetereoaromatics and carbanions.
Chlorination reagents can be activated by strong acids, Lewis acids and oxidants. Oxidants can optimize atom efficiency by generating Cl2/Cl+ in situ from simple chloride salts. Oxidants can also reoxidize any Cl– bi-product and maximize the use of chlorine. Apart from the generation of carbon–chlorine bonds, Cl2 is often used for the cleavage of disulphides and sulphides to generate sulphonyl chlorides.
(re)Oxidants used in chlorination
H2O2 or urea H2O2 complex
O2
Oxone KHSO5 0.5KHSO4 0.5K2SO4
Acids used to promote chlorination
HOAc
Oleum
H2SO4
Chlorination using Cl2 or Cl2 generated in situ is normally the most atom-efficient and sustainable chlorination
chemistry. Other common reagents can be classified as below:
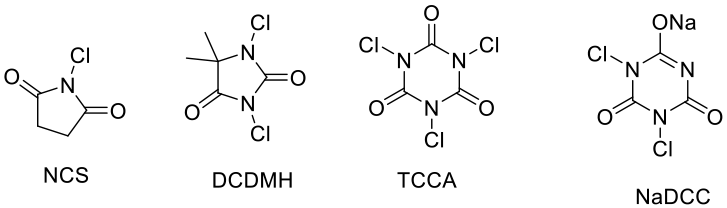
- N- Cl compounds
NCS, DCDMH, TCCA , NaDCC – These can act as sources of electrophilic chlorine and radical chlorine.
- Hypervalent chlorine compounds: NaOCl, NaClO3
- Polychlorinated materials can generate a good leaving group on donation of Cl+ – CCl4, C2Cl6. This reagent class generally needs activation with a phosphine or a reaction with a powerful nucleophile – RLi, RMgCl, etc.
-
Atom efficiency (by-products Mwt)
NaOCl, HCl Na/KCl salts, and Cl2 with activators/(re)oxidants are the most atom-efficient and maximize the use of chlorine. The use of other Cl+ reagents is less atom-efficient. - Safety Concerns
Chlorine gas is highly reactive and toxic and special precautions need to be taken to handle this material.
Saroha, A. K. Safe Handling of Chlorine. Chemical Health & Safety. 2006, 13(2) , 5-11.
Gustin, J. Safety of Chlorine Production and Chlorination Processes. Chemical Health & Safety. 2005, 12(1), 5-16.
Chlorination (electrophilic and radical) reactions can be very exothermic, especially when activators and oxidants are added. Care should be taken with solvent compatibility (see solvents section). The operational hazards associated with electrophilic and radical chlorination can be somewhat mitigated by the use of flow chemistry. High valency chlorine compounds and N-Cl compounds can cause fire and may be explosive in combination with other materials.
NaOCl can be explosive in combination with some organic compounds, especially amines and ammonia, or ammonia sources that can generate NCl3.
Cl2 is highly corrosive and most chlorinating reagents will generate some HCl, so the material’s compatibility needs consideration before scale up. - Toxicity and environmental/aquatic impact
High concentrations of Cl2 and Cl+ reagents will be extremely toxic to aquatic organisms, but are too reactive to be persistent in the environment – most are gross cellular poisons. Longer-term environmental effects will reflect organic materials associated with the reagent. High chloride content aqueous waste can be deleterious to fresh water aquatic life forms.
Higher molecular weight organic cations can be inhibitory or toxic to certain aquatic life forms, so caution needs to be exercised with aqueous wastes.
Polychlorinated organics can be persistent and bioaccumulate.
C2Cl6 is a persistent organic pollutant (POP).
CCl4 is regulated under the Montreal protocol. - Cost, availability & sustainable feedstocks
Most chlorination reagents are available at scale with varying degrees of cost, typically much cheaper than the corresponding bromine-based reagents. - Sustainable implications
No real sustainability issues with chlorine
Key references
Relevant scale up example
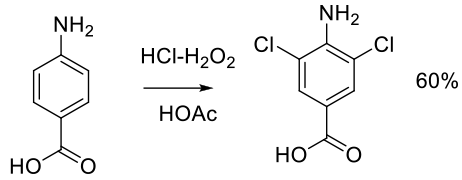
Org. Process Res. Dev. 1999, 3, 10-16.
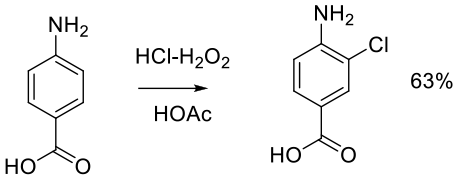
Org. Process Res. Dev. 1999, 3, 10-16.
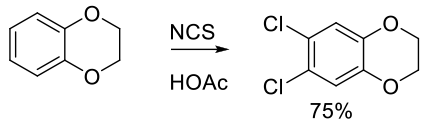
Org. Proc. Res. Dev. 1999, 3, 196-200.
Experimental
25 kg scale
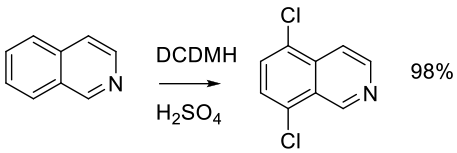
Org. Proc. Res. Dev. 2010, 14, 108–113.
Experimental
400 g scale
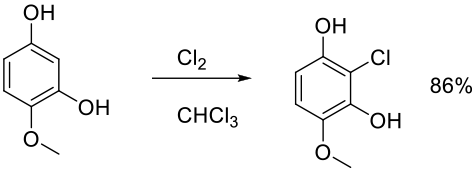
Org. Proc. Res. Dev. 2010, 14, 108–113.
Experimental
100 g scale
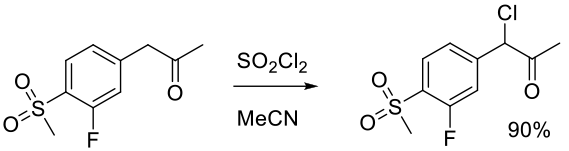
Org. Process Res. Dev. 2008, 12, 111–115.
Experimental
23 g scale
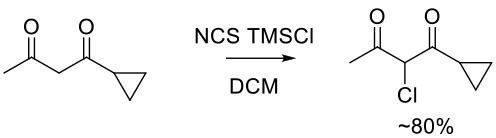
Org. Process Res. Dev. 2009, 13, 848–853.
Experimental
9 kg scale
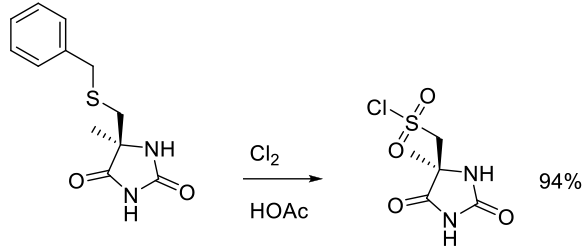
Org. Process Res. Dev. 2010, 14, 278–288.
Experimental
2.8 kg scale
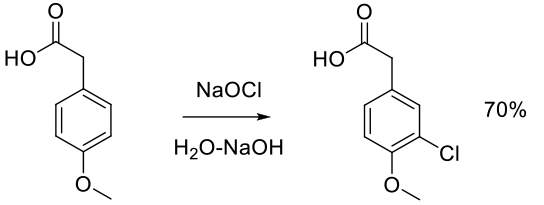
Org. Process Res. Dev. 1997, 1, 359-364.
Experimental
100 g scale
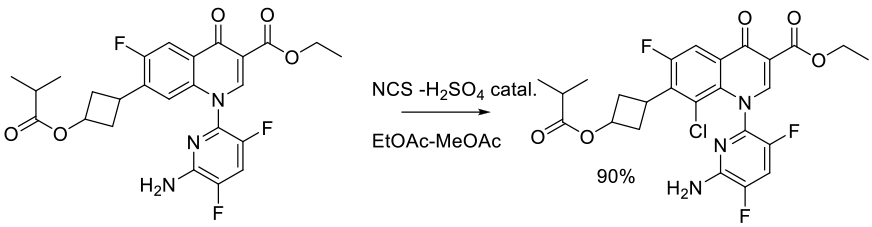
Org. Process Res. Dev. 2006, 10, 751-756.
Experimental
93 g scale
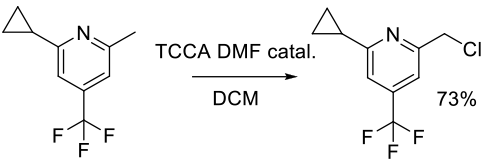
Org. Process Res. Dev. 2013, 17, 257−264.
Experimental
70 g scale
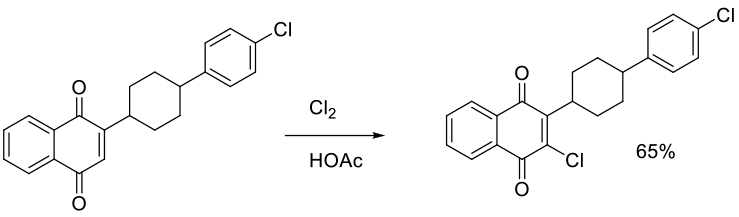
Org. Process Res. Dev. 2014, 18, 618−625.
Experimental
6 g scale
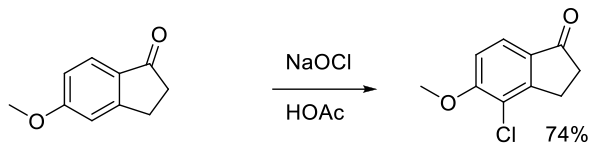
Org. Process Res. Dev. 2008, 12, 723–730.
Experimental
18 kg scale