Nucleophilic Cl– Reagents
Mechanism + Description
Typically SN2 with displacement of another halogen (Iodide) or leaving group (sulphonate, nitro), etc. Also via reaction of Cl– with other electrophiles, like diazonium salts. Chlroride anions can add to radical iminium cations as well.
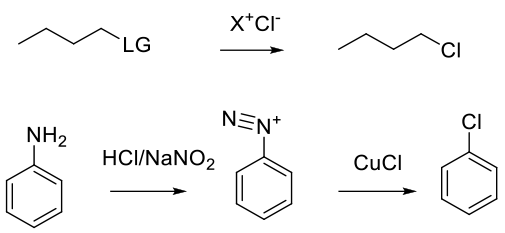
General comments
Normally, simple bromide salts like Na/K/Li Chloride, or tetralkyl ammonium or phosphonium chlorides like nBu4NCl, are used. Hydrogen chloride is typically used for deoxychlorination, but can be used to add HCl to unsaturated C-C bonds and epoxides, etc. The Sandmeyer reaction can be used to make aryl chlorides from anilines.
Key references
Relevant scale up example
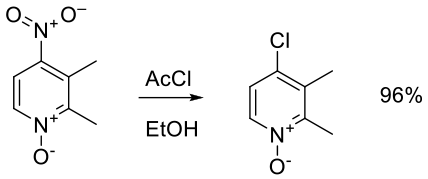
Org. Process Res. Dev. 2010, 14, 562–567.
Experimental
26 kg scale