Carbon-Centred Fluorination Reagents
Mechanism + Description
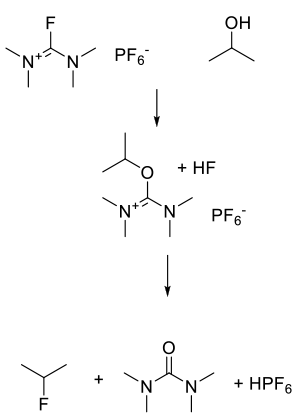
These reagents activate the alcohol forming a good leaving group, which is then displaced in an SN2 fashion by fluoride.
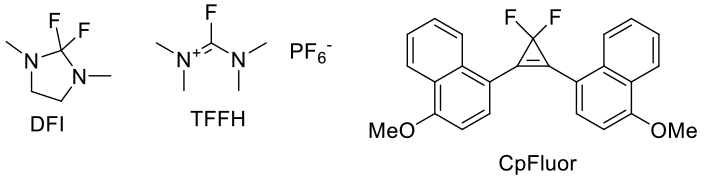
General comments
These reagents are less reactive than S-based deoxyfluorination reagents and convert alcohols to fluorides and acids to acid fluorides. Their reactivity is similar to the Ishikawa’s reagent and related perfluoroalkanes. Some of these reagents need catalysis by HF complexes. The use of these reagents at scale maybe limited by commercial availability. They all show poor atom economy, but have the potential of selective fluorination of alcohols in the presence of other reactive groups like ketones.
Key references
Hawkins, J. M.; Dubé, P.; Maloney, M. T.; Wei, L.; Ewing, M.; Chesnut, S. M.; Denette, J. R.; Lillie, B. M.; Vaidyanathan, R. Org. Process Res. Dev. 2012, 16, 1393-1403. Note: Use of DFI and TFFH
Relevant scale up examples
None found.