Fluorine F2
Mechanism + Description
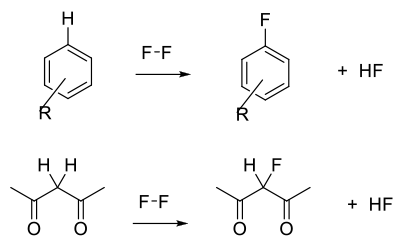
F2 is an electrophilic reagent normally used to add to unsaturated C-C bonds, take part in electrophilic substitution reactions with aromatics/heteroaromatics, or react with acidic C-H bonds.
General comments
Fluorine gas is highly toxic, highly corrosive and incompatible with many materials including glass. For more information see EROS Fluorine entry. Engineering solutions sometimes allow the health and safety risks of Fluorine or HF to be mitigated on large scale. Hence in a few specific instances the atom efficiency of fluorine can paradoxically lead to it being the greenest choice. This assessment however covers all scales of usage and such specialized engineering approaches are not factored in. A safer option is 3-20% F2 diluted with N2. The high reactivity of F2 often results in poor selectivity, over fluorination, and oxidation.
Key references
Chambers, R. D.; Skinner, C. J.; Atherton, M. J.; Moilliet, J. S. Elemental Fluorine. Part 4. Use of Elemental Fluorine for the Halogenation of Aromatics. J. Chem. Soc., Perkin Trans. 1,1996,14, 1659-1664.
Sandford, G. Elemental Fluorine in Organic Synthesis. J. Fluorine Chem.2007, 128 (2), 90-104.
Purrington, S. T.; Kagen, B. S.; Patrick, T. B. Application of elemental fluorine in organic synthesis. Chem. Rev. 1986, 86, 997–1018.
Harsanyi, A.; Sandford, G. 2-Fluoromalonate Esters: Fluoroaliphatic Building Blocks for the Life Sciences. Org. Process Res. Dev. 2014,18, 981–992.
McPake, C. B.; Sandford, G. Selective Continuous Flow Processes Using Fluorine Gas. Org. Process Res. Dev.2012, 16, 844–851.
Relevant scale up example
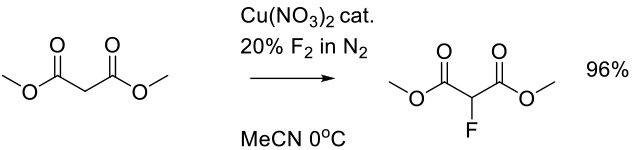
Harsanyi, A.; Sandford, G. Green Chem. 2015, 17, 3000-3009.
Experimental
40 g scale
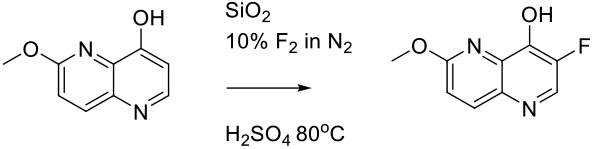
Abele, S.; Schmidt, G.; Fleming, M. J.; Steiner, H. Org. Process Res. Dev.2014,18, 993–1001.
Experimental
50 g scale

Harsanyi, A.; Conte, A.; Pichon, L.; Rabion, A.; Grenier, S.; Sandford, G. Org. Process Res. Dev. 2017, 21, 273-276.
Experimental
~ 60 g/h scale
Green Review
-
Atom efficiency (by-products Mwt)
Introduction of fluorine by F2 is the most atom efficient route to fluorinated molecules giving just HF (19) as a by-product. In principal, the waste HF could be recycled. - Safety Concerns
F2 and F2–N2 mixtures are extremely dangerous with respect to corrosion, reactivity and acute toxicity. Any liberated HF can cause severe burns. F2 is a strong oxidizer and can react violently with organic materials. Hazards associated with F2 chemistry can be somewhat mitigated by using flow processing.
Toxicity of HF Toxicity of F2 - Toxicity and environmental/aquatic impact
F2 is too reactive to persist in the environment. High concentrations of F2/HF are extremely toxic to all life forms. Lower concentrations of fluoride can be harmful to aquatic life. Organofluorine compounds, especially polyfluorinated, can be persistent and bioaccumulate.
Fluoride toxicity to aquatic organisms: a review. - Cost, availability & sustainable feedstocks
F2 and F2–N2 mixtures are available in bulk. - Sustainable implications
Fluoride sources are at medium risk of depletion and all fluorine is used in a dispersive manner. The high energy needed to produce F2 would make this a high LCI impact material.