Mechanism + Description
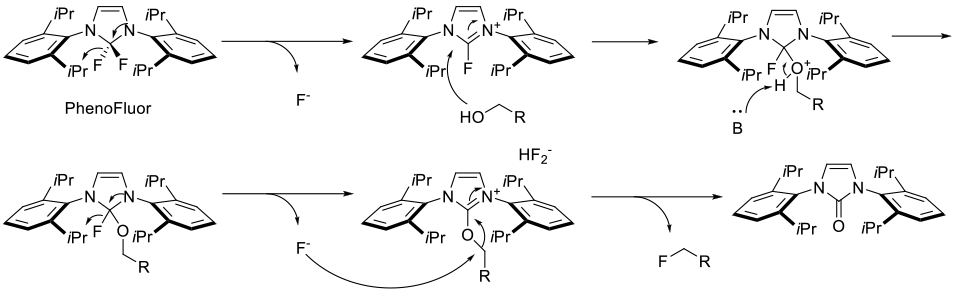
PhenoFluor serves to activate the alcohol/phenol forming a alkoxy-/phenoxy-imidazolium bifluoride salt, which can be displaced in an SN2 fashion by fluoride.
General comments
PhenoFluor is a unique reagent in being able to mediate the deoxyfluorination of both alcohols and phenols depending on the conditions employed. It has been utilized for the late stage deoxyfluorination of a series of complex bioactive substances with good functional group tolerance, and predictable reactivity for poly-hydroxylated system. It’s use on scale may be limited due to lack of bulk commercial availability. In addition, the reagent is poor from an atom economy standpoint, and displays a degree of moisture sensitivity, though the latter issue has been mitigated by the development of alternative formulations.
Key references
Tang, P.; Wang, W.; Ritter, T. Deoxyfluorination of Phenols. J. Am. Chem. Soc. 2011,133, 11482–11484.
Fujimoto, T.; Becker, F.; Ritter, T. PhenoFluor: Practical Synthesis, New Formulation, and Deoxyfluorination of Heteroaromatics. Org. Process Res. Dev. 2014, 18, 1041-1044.
Sladojevich, F.; Arlow, S. I.; Tang, P.; Ritter, T. Late-Stage Deoxyfluorination of Alcohols with PhenoFluor. J. Am. Chem. Soc. 2013, 135, 2470-2473.
Fujimoto, T.; Ritter, T. PhenoFluorMix: Practical Chemoselective Deoxyfluorination of Phenols. Org. Lett. 2015, 17, 544-547.
Goldberg, N. W.; Shen, X.; Li, J.; Ritter, T. AlkylFluor: Deoxyfluorination of Alcohols. Org. Lett. 2016, 18, 6102-6104.
Neumann, C. N.; Ritter, T. Facile C-F Bond Formation through a Concerted Nucleophilic Aromatic Substitution Mediated by the PhenoFluor Reagent. Acc. Chem. Res. 2017, 50, 2822-2833.
Hu, W.-L.; Hu, X.-G.; Hunter, L. Recent Developments in the Deoxyfluorination of Alcohols and Phenols: New Reagents, Mechanistic Insights, and Applications. Synthesis 2017, 49, 4917-4930.
Relevant scale up examples
None found.
