‘One Pot’ in situ Borylation by C-H Activation
Mechanism + Description
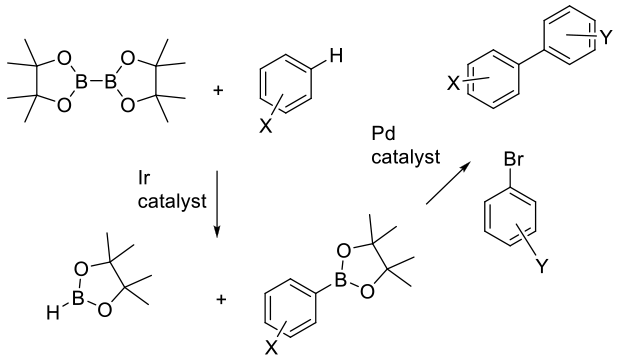
The Boron coupling partner is formed in situ via a C-H activation reaction catalyzed by an iridium catalyst. The reaction then occurs with the aryl/heteroaryl halide/Pd catalyst via the usual Suzuki mechanism.
General Comments
The recent development Miller and Hartwig (are these the scientists who made the ‘development’?) of Ir-based catalytic systems that enables the insertion of pinacolylborane directly into an unactivated Ar-H bond has opened up the exciting prospect of a much greener Suzuki reaction by obviating the need to prepare the arylboron intermediate from an aryl halide. The least sterically hindered Ar-H bond is activated (for example, the 5 position of a 1,3-disubstituted benzene ring regardless of the electronics of the substituents), which gives access to previously highly inaccessible substitution patterns. The telescoping of this reaction with the Suzuki coupling is still in its infancy, but it is an area of very active pursuit.
Key References
Relevant Scale-Up Examples with Scheme
None located.