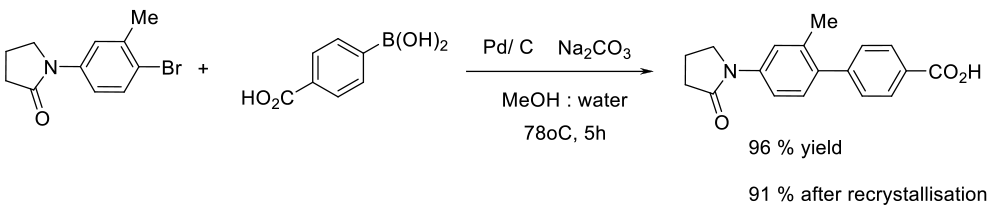Mechanism + Description
See soluble catalysts – mechanism may be on the catalyst surface or via a low standing level of a
soluble Pd complex
General comments
One of the greenest ways of performing a Suzuki reaction is to use a heterogeneous procedure using Pd on carbon without a ligand. These catalysts are typically employed for hydrogenation reactions and are thus readily available at scale. The palladium can be recovered by simple filtration. These Suzuki couplings are often carried out in water or water-alcohol mixtures. Use of an aqueous solvent mixture minimizes the risks of handling the pyrophoric catalyst. The use of Pd/C typically results in low levels of leaching from the Pd catalyst into the product; most papers report 20 ppm or less.
A range of other heterogeneous Pd catalysts for Suzuki couplings are also available commercially or easily prepared (see references). It should never be assumed that there is no Pd leaching from these heterogeneous catalysts until proven analytically. Metal leaching depends on the catalyst, solvents, and the ligating power of the reagents/substrates/products. There is growing interest in highly active nanoparticulate catalysts.
Key references
Paul, S.; Islam, M.; Islam, M. Suzuki–MiyauraReaction by Heterogeneously Supported Pd in Water: Recent Studies. RSC Adv. 2015, 5, 42193-42221.
Jiang, J.; Sclafani, J.; Prasad, K.; Repič, O.; Blacklock, T. J. Pd−Smopex-111: A New Catalyst for Heck and Suzuki Cross-Coupling Reactions. Org. Process Res. Dev. 2007, 11 (4), 769–772.
Ciriminna, R.; Pandarus, V.; Fidalgo, A.; Ilharco, L. M.; Béland, F.; Pagliaro, M. SiliaCat: A Versatile Catalyst Series for Synthetic Organic Chemistry. Org. Process Res. Dev. 2015, 19 (7), 755–768.
Marck, G.; Villiger, A.; Buckhecker, R. Aryl Couplings with Heterogeneous Palladium Catalysts. Tetrahedron Lett. 1994, 35 (20), 3277–3280.
Yin, L.; Liebscher, J. Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107 (1), 133–173.
Felpin, F.; Ayad, T.; Mitra, S. Pd/C: An Old Catalyst for New Applications – Its Use for the Suzuki–MiyauraReaction. Eur. J. Org. Chem. 2006, 2006 (12), 2679–2690.
Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H. A Highly Active Heterogeneous Palladium Catalyst for the Suzuki–Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew. Chem., Int. Ed. 2010, 49 (24), 4054-4058.
Li, Y.; Hong, X. M.; Collard, D. M.; El-Sayed, M. A. Suzuki Cross-Coupling Reactions Catalyzed by Palladium Nanoparticles in Aqueous Solution. Org. Lett. 2000, 2 (15), 2385–2388.
Susanto, W.; Chu, C.; Ang, W. J.; Chou, T.; Lo, L.; Lam, Y. Fluorous Oxime Palladacycle: A Precatalyst for Carbon–Carbon Coupling Reactions in Aqueous and Organic Medium. J. Org. Chem. 2012, 77 (6), 2729–2742.
FF
Samarasimhareddy, M.; Prabhu, G.; Vishwanatha, T. M.; Sureshbabu, V. V. PVC-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki Cross-Coupling Reactions at Room Temperature. Synthesis. 2013, 45 (9), 1201-1206.
Susanto, W.; Chu, C.; Ang, W. J.; Chou, T.; Lo, L.; Lam, Y. Fluorous Oxime Palladacycle: A Precatalyst for Carbon–Carbon Coupling Reactions in Aqueous and Organic Medium. J. Org. Chem. 2012, 77 (6), 2729–2742.
Key references
Paul, S.; Islam, M.; Islam, M. Suzuki–MiyauraReaction by Heterogeneously Supported Pd in Water: Recent Studies. RSC Adv. 2015, 5, 42193-42221.
Jiang, J.; Sclafani, J.; Prasad, K.; Repič, O.; Blacklock, T. J. Pd−Smopex-111: A New Catalyst for Heck and Suzuki Cross-Coupling Reactions. Org. Process Res. Dev. 2007, 11 (4), 769–772.
Ciriminna, R.; Pandarus, V.; Fidalgo, A.; Ilharco, L. M.; Béland, F.; Pagliaro, M. SiliaCat: A Versatile Catalyst Series for Synthetic Organic Chemistry. Org. Process Res. Dev. 2015, 19 (7), 755–768.
Marck, G.; Villiger, A.; Buckhecker, R. Aryl Couplings with Heterogeneous Palladium Catalysts. Tetrahedron Lett. 1994, 35 (20), 3277–3280.
Yin, L.; Liebscher, J. Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107 (1), 133–173.
Felpin, F.; Ayad, T.; Mitra, S. Pd/C: An Old Catalyst for New Applications – Its Use for the Suzuki–MiyauraReaction. Eur. J. Org. Chem. 2006, 2006 (12), 2679–2690.
Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H. A Highly Active Heterogeneous Palladium Catalyst for the Suzuki–Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew. Chem., Int. Ed. 2010, 49 (24), 4054-4058.
Li, Y.; Hong, X. M.; Collard, D. M.; El-Sayed, M. A. Suzuki Cross-Coupling Reactions Catalyzed by Palladium Nanoparticles in Aqueous Solution. Org. Lett. 2000, 2 (15), 2385–2388.
Susanto, W.; Chu, C.; Ang, W. J.; Chou, T.; Lo, L.; Lam, Y. Fluorous Oxime Palladacycle: A Precatalyst for Carbon–Carbon Coupling Reactions in Aqueous and Organic Medium. J. Org. Chem. 2012, 77 (6), 2729–2742.
FF
Samarasimhareddy, M.; Prabhu, G.; Vishwanatha, T. M.; Sureshbabu, V. V. PVC-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki Cross-Coupling Reactions at Room Temperature. Synthesis. 2013, 45 (9), 1201-1206.
Susanto, W.; Chu, C.; Ang, W. J.; Chou, T.; Lo, L.; Lam, Y. Fluorous Oxime Palladacycle: A Precatalyst for Carbon–Carbon Coupling Reactions in Aqueous and Organic Medium. J. Org. Chem. 2012, 77 (6), 2729–2742.
Relevant Scale-Up Examples with Scheme
Org. Process Res. Dev. 2009, 13 (2), 268–275.
Experimental
50 g scale
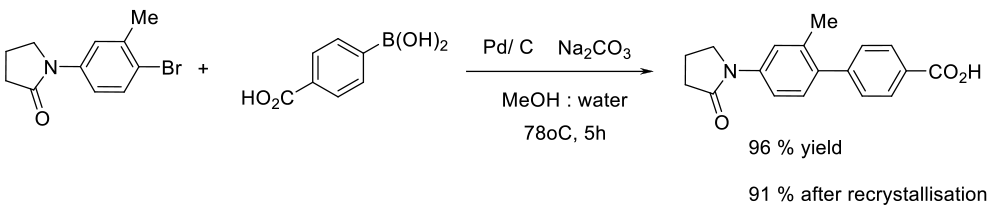
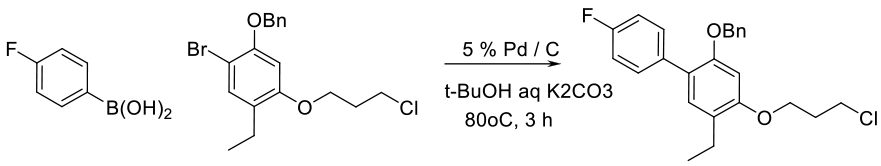
Org. Process Res. Dev. 1999, 3 (4), 248–252.
Experimental
6 kg scale
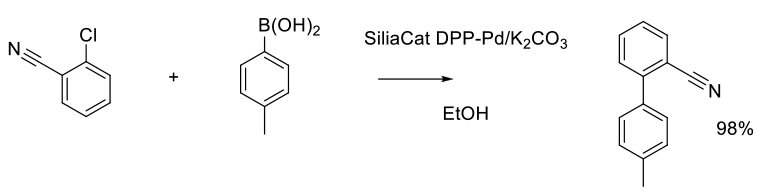
Org. Process Res. Dev. 2013, 17 (12), 1492−1497.
Experimental
100 g scale
Org. Process Res. Dev. 2006, 10 (1), 36-45.
Experimental
1.7 kg scale