Mechanism + Description
Use of base metal complexes to replace Pd – mechanism as for Pd coupling
General comments
A number of precious and base metals have been reported to replace Pd in the Suzuki reaction. These include Ru, Ni, Fe and Cu. Nickel is probably the most studied replacement metal. Base metals are typically used at higher loadings than Pd metal complexes. This is adequately compensated for by their much lower cost, increased abundance, and lower life cycle impact.
A degree of caution needs to be exercised when working with base metals and other reaction conditions claiming to be ‘metal free’ since the Pd contamination of equipment, even at very low ppm levels, or other added inorganic reagents can produce an active catalyst for Suzuki reactions.
Leadbeater, N. E.; Marco, M. Transition-Metal-Free Suzuki-Type Coupling Reactions. Angew. Chem., Int. Ed. 2003, 42 (12), 1407-1409.
Key references
Mesganaw, T.; Garg, N. K. Ni- and Fe-Catalyzed Cross-Coupling Reactions of Phenol Derivatives. Org. Process Res. Dev. 2013, 17 (1), 29−39.
Gurung, S. K.; Thapa, S.; Kafle, A.; Dickie, D. A.; Giri, R. Copper-Catalyzed Suzuki−Miyaura Coupling of ArylboronateEsters:Transmetalation with (PN)CuF and Identification of Intermediates. Org. Lett. 2014, 16 (4), 1264–1267.
Lee, C.; Ke, W.; Chan, K.; Lai, C.; Hu, C.; Lee, H. M. Nickel(II) Complexes of Bidentate N-Heterocyclic Carbene/Phosphine Ligands: Efficient Catalysts for Suzuki Coupling of Aryl Chlorides. Chem. Eur. J. 2007, 13 (2), 582-591.
Saito, B.; Fu, G. C. Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2007, 129 (31), 9602-9603.
Han, F. Transition-Metal-CatalyzedSuzuki–MiyauraCross-Coupling Reactions: A Remarkable Advance from Palladium to Nickel Catalysts. Chem. Soc. Rev. 2013, 42 (12), 5270-5298.
Bedford, R. B.; Hall, M. A.; Hodges, G. R.; Huwe, M.; Wilkinson, M. C. Simple Mixed Fe–Zn Catalysts for the Suzuki Couplings of Tetraarylborateswith Benzyl Halides and 2-Halopyridines. Chem. Commun. 2009, 0 (42), 6430-6432.
Hatakeyama, T.; Hashimoto, T.; Kathriarachchi, K. K. A. D. S.; Zenmyo, T.; Seike, H.; Nakamura, M. Iron-Catalyzed Alkyl-Alkyl Suzuki-MiyauraCoupling. Angew. Chem., Int. Ed. 2012, 51 (35), 8834–8837.
Relevant Scale-Up Examples with Scheme
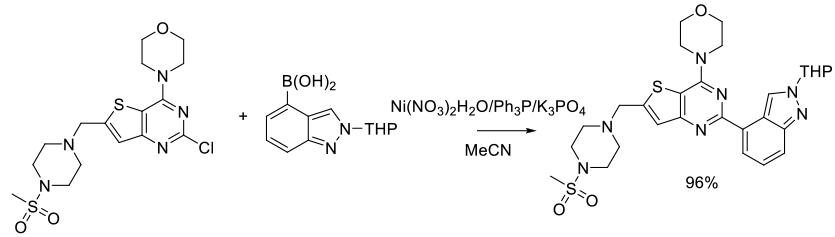
Org. Process Res. Dev. 2013, 17, 97−107
Experimental
48 kg scale
