Meerwein-Ponndorf-Verley Reduction
Mechanism + Description
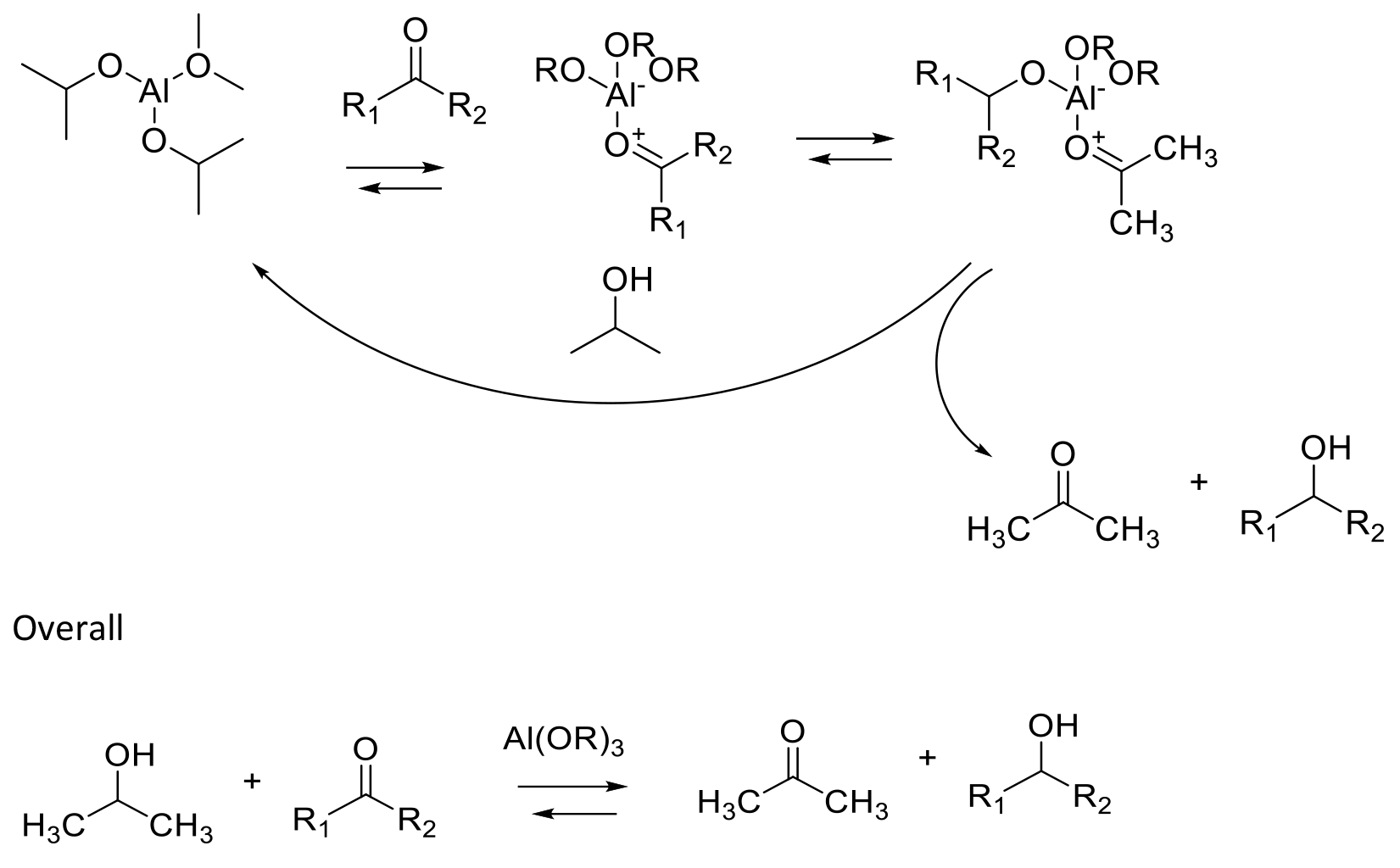
A hydrogen transfer reaction using aluminium alkoxide as a catalyst. The reaction mechanism of Meerwein-Ponndorf-Verley (MPV) reactions proceeds in most cases via a cyclic six-membered transition state in which both reductant and oxidant are coordinated to the metal centre of a metal alkoxide catalyst. The reaction is driven towards the most thermodynamically stable product and by the use of an excess of the sacrificial H2 source (usually isopropanol) and removal of acetone. This reaction if driven in the oxidation direction is known as the Oppenauer oxidation.
General comments
MVP reduction has a reputation as an old fashioned methodology that has been superseded by more modern reagents. But the reaction is very green just using catalytic amounts of Al(OiPr)3 as base with isopropanol the only by-product. The reaction can also used on a large scale. The reaction can be driven in oxidation or reduction mode using an excess of simple alcohols or ketones as donors or acceptors. A detraction is that sometimes large amounts of Al(OiPr)3 have to be used. This is due to the polymeric nature of bulk Al(OiPr)3 rendering it kinetically slow. Other Al3+ complexes like Al(OtBu)3 can be employed as more efficient catalysts, and Al alkoxides generated in situ from Al(Me)3 usually show much faster kinetics than commercial Al alkoxides. Other metals like Fe3+ can be used as catalysts in MVP reduction.
In the presence of a directing existing chiral centre, diastereomeric reduction can be achieved. In the presence of chiral ligands, enantioselective reduction can occur, but it is more usual to see this transformation done with Ru, Rh or Ir chiral catalysts.
Key references
Relevant scale up examples
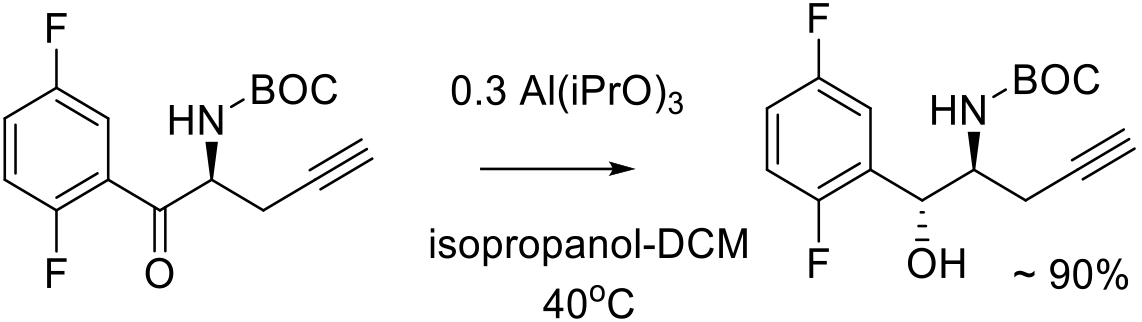
Org. Proc. Res. Dev. 2016, 20, 2074−2079.
900 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Atom efficiency depends on sacrificial ketone used and how many equivalents needed. It can be good provided a large excess of acceptor is not needed to drive the equilibrium. - Safety Concerns
No major concerns. - Toxicity and environmental/aquatic impact
Aluminium wastes are manageable, especially if used in catalytic quantities. - Cost, availability & sustainable feedstocks
Materials readily available and cheap. - Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Aluminium is currently listed as being at low risk of depletion. Used catalytically, recycling may not be economically feasible.